Cancer antigens and utilization thereof
a cancer antigen and human cancer technology, applied in the field of human cancer antigens, can solve the problems of large number of advanced cancers that have not yet been treated, lack of anti-tumor effects, and difficulty in identifying tumor antigens from carcinomas other than melanomas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0088] The serum was collected from a patient with pancreatic cancer. The collected serum was conserved at −80° C. From this serum sample, an antibody reacting with Escherichia coli and λ phage was eliminated by using a column that was filled with dissolved matter of Escherichia coli and phage and sepharose 4B. Thereafter, the resultant serum was 100 to 800 times diluted and then used.
[0089] A phage cDNA library produced by inserting cDNA of a pancreatic cancer cell line CFPAC-1 into a λZAP express vector was purchased from Stratagene, La Jolla, Calif. Escherichia coli was infected with this λ phage cDNA library, and it was then cultured on NZY plate medium at 42° C. for 6 hours, so as to produce plaques. Thereafter, the plate was covered with a nitrocellulose membrane into which isopropyl β-D-thiogalactoside (IPTG) had been infiltrated at 37° C. for 3 hours, so as to produce a protein encoded by the cDNA that had been incorporated into λ phage in the plaques.
[0090] The protein pr...
example 2
[0094] A motif binding to HLA-A24, for which 60% of Japanese people get positive, is identical to a motif, to which Kd of a BALB / c mouse binds. A peptide that is shared by human hsp105 and mouse hsp105 and is predicted to bind to both HLA-A24 and Kd is selected from the sequence of hsp105, using HLA-peptide binding prediction (http: / / bimas / dcrt.nih.gov / molbio / hla_bind / ). Nine types of peptides consisting of 9 or 10 amino acids were synthesized by the Fmoc / PyBOP method. The sequences of the peptides and the estimated binding values to Kd are shown in Table 2.
TABLE 2hsp105-derived peptideshsp105-derived peptidesNo.PositionSequenceBinding Score1hsp105 180-188NYGIYKQDL24002hsp105 214-223AFNKGKLKVL9603hsp105 251-260KYKLDAKSKI28804hsp105 305-313QFEELCAEL13825hsp105 433-442TFLRRGPFEL19206hsp105 570-579MYIETEGKMI48007hsp105 597-606ECVYEFRDKL808hsp105 682-690HYAKIAADF609hsp105 696-705KYNHIDESEM432
example 3
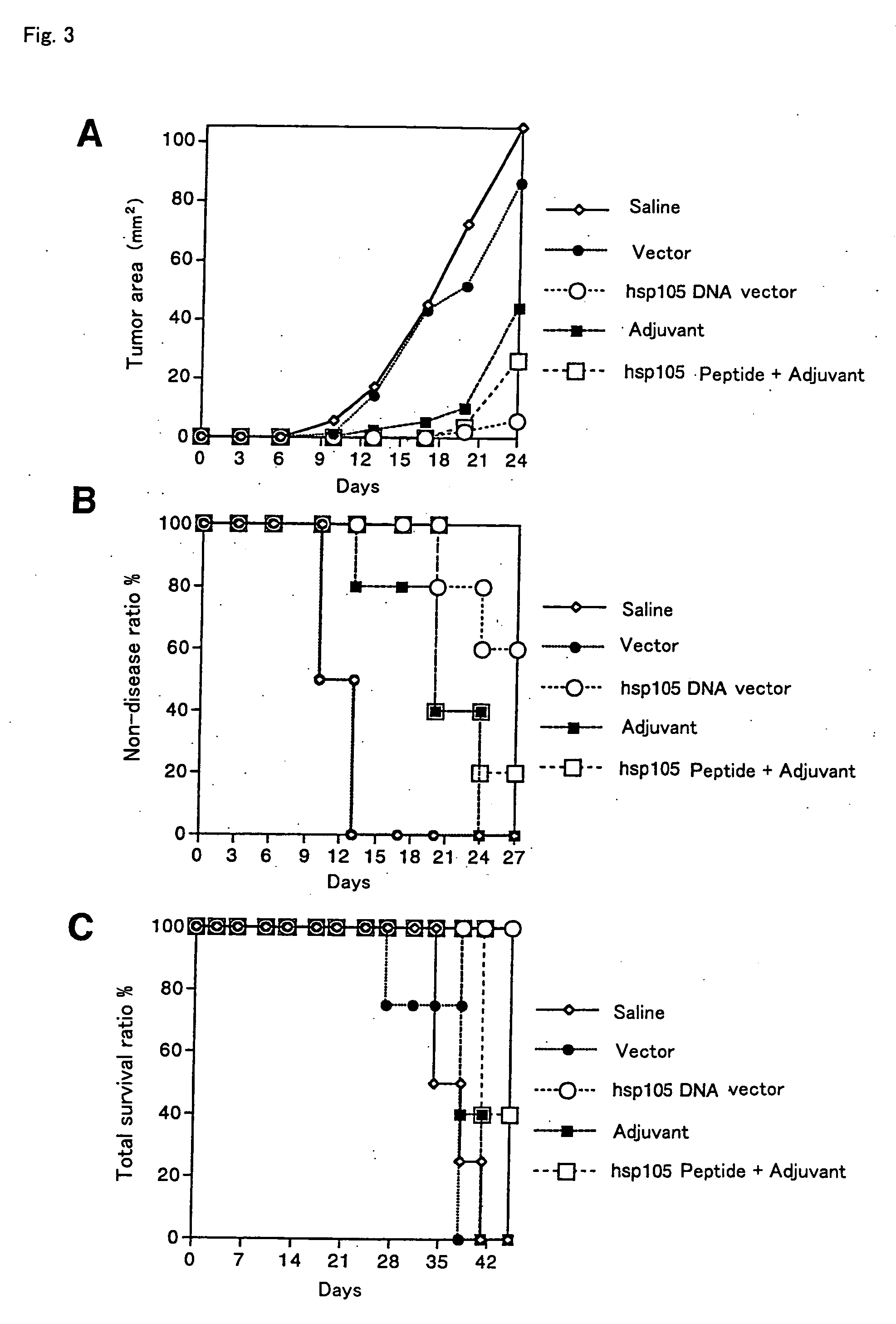
[0095] Plasmid DNA produced by incorporating mouse hsp105 cDNA into an expression vector pCAGGS was adjusted to be a suitable concentration. It was then used as a vaccine for the following performance evaluation test. With regard to this mouse hsp105-pCAGGS DNA vaccine, Escherichia coli was cultured, and thereafter, plasmid DNA was extracted from the Escherichia coli and purified, so as to produce the vaccine in large scale.
[0096] The following samples were injected into the muscle of each BALB / c mouse: (1) a normal saline solution, (2) only a vector, (3) a hsp105 cDNA vector, (4) only an adjuvant, (5) an adjuvant +a peptide. Thereafter, a colon cancer cell line Colon-26 derived from a syngeneic mouse, that highly expresses hsp105, was subcutaneously transplanted into the back of the mouse. Thereafter, the development of the cancer in the mice was evaluated in the following points: (1) the area of a cancerous portion, (2) the ratio of mice in which the cancer developed, and (3) the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com