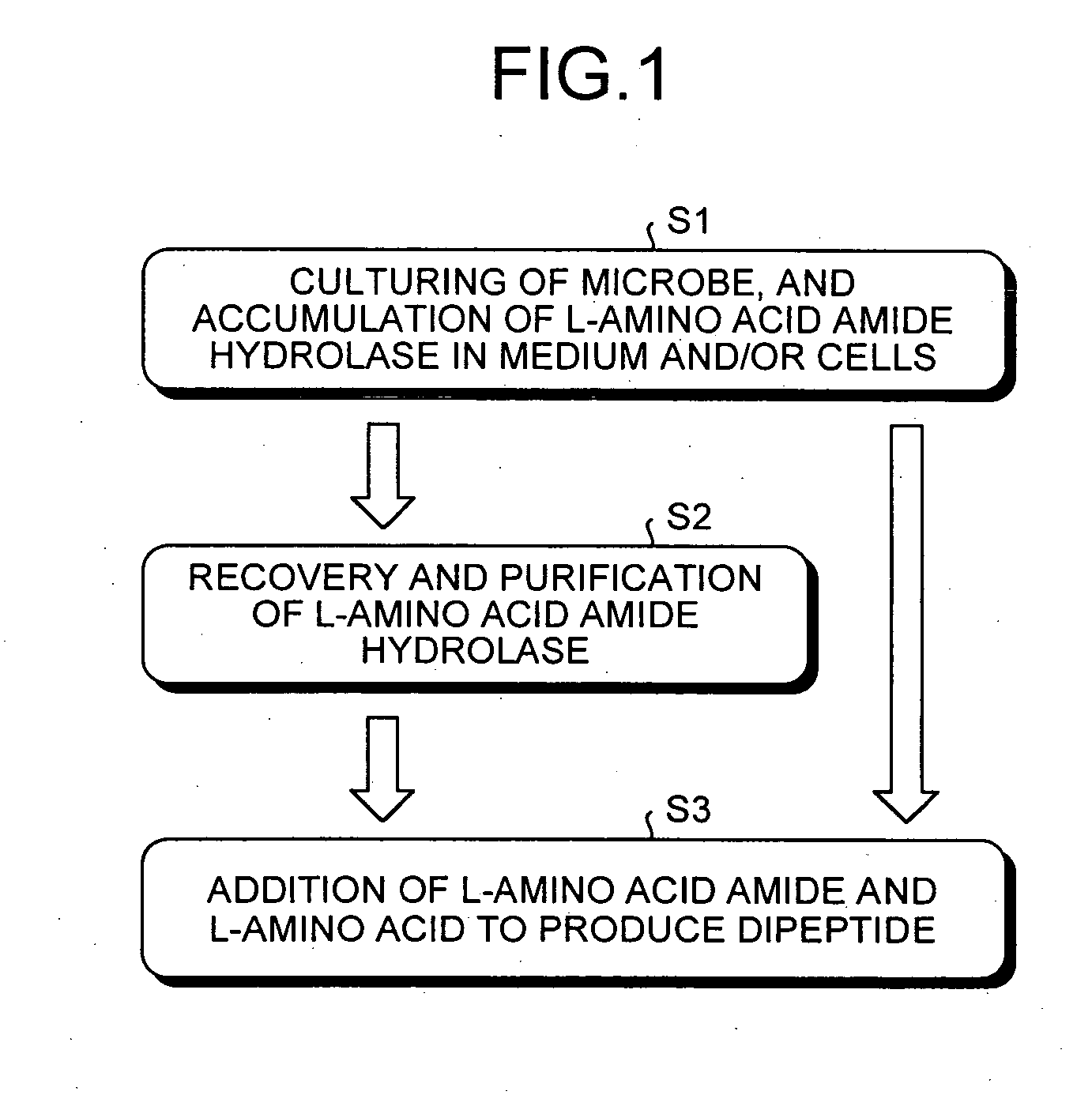
Dipeptide production method, L-amino acid amide hydrolase used therein, and production method of L-amino acid amide hydrolase
a technology of l-amino acid amide hydrolase and production method, which is applied in the direction of peptides, enzymology, biochemistry apparatus and processes, etc., can solve the problems of not being adequately satisfactory in terms of industrial advantages and difficult production methods, and achieve simple production pathway and low cost. , the effect of easy availability of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of L-Alanyl-L-Asparagine
[0108] A 50 milliliter (ml or mL) aliquot of a medium containing 0.5% (w / v) yeast extract, 0.5% (w / v) peptone, 0.5% (w / v) glycerol, 0.5% (w / v) sodium chloride and 0.5% (w / v) L-alanine amide hydrochloride (pH 7.0) was dispensed to a 500 mL Sakaguchi flask and sterilized at 120° C. for 20 minutes. One loopful of the microbes shown in Table 1, which were cultured at 30° C. for 24 hours on a slant medium containing 0.5% (w / v) yeast extract, 0.5% (w / v) peptone, 0.5% (w / v) glycerol, 0.5% (w / v) sodium chloride, 0.5% (w / v) L-alanine amide hydrochloride and 2% (w / v) agar (pH 7.0), was inoculated into the aforementioned medium and cultured by shake culturing for 20 hours at 30° C. and 120 strokes / minute. Following the culturing, the microbial cells were separated by centrifugation, washed twice with an amount of physiological saline equal to the amount of culture liquid and centrifuged again to collect the microbial cells followed by suspending with 0.2 M T...
example 2
Purification of L-Alanine Amide Hydrolase from Corynebacterium glutamicum Strain ATCC 13286
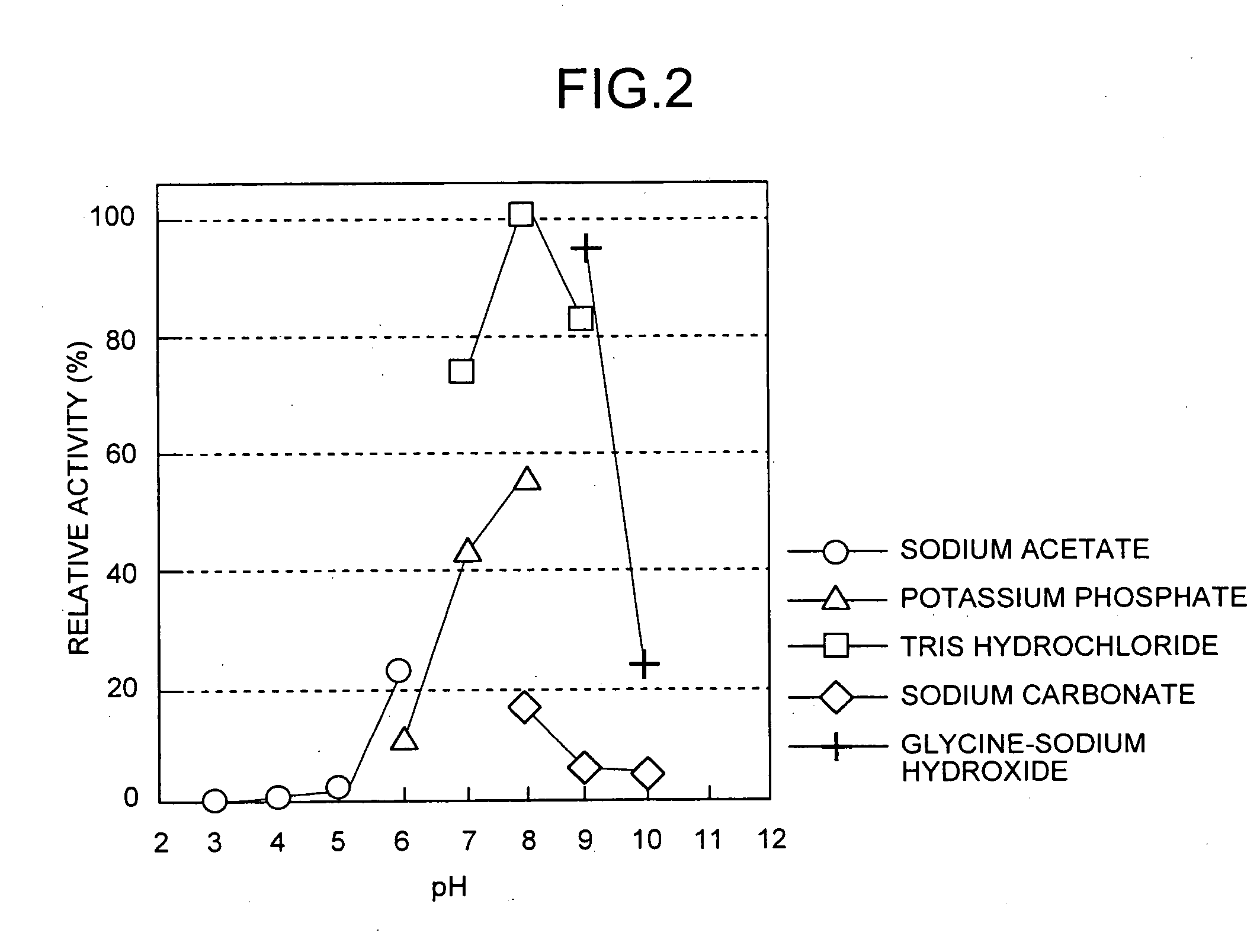
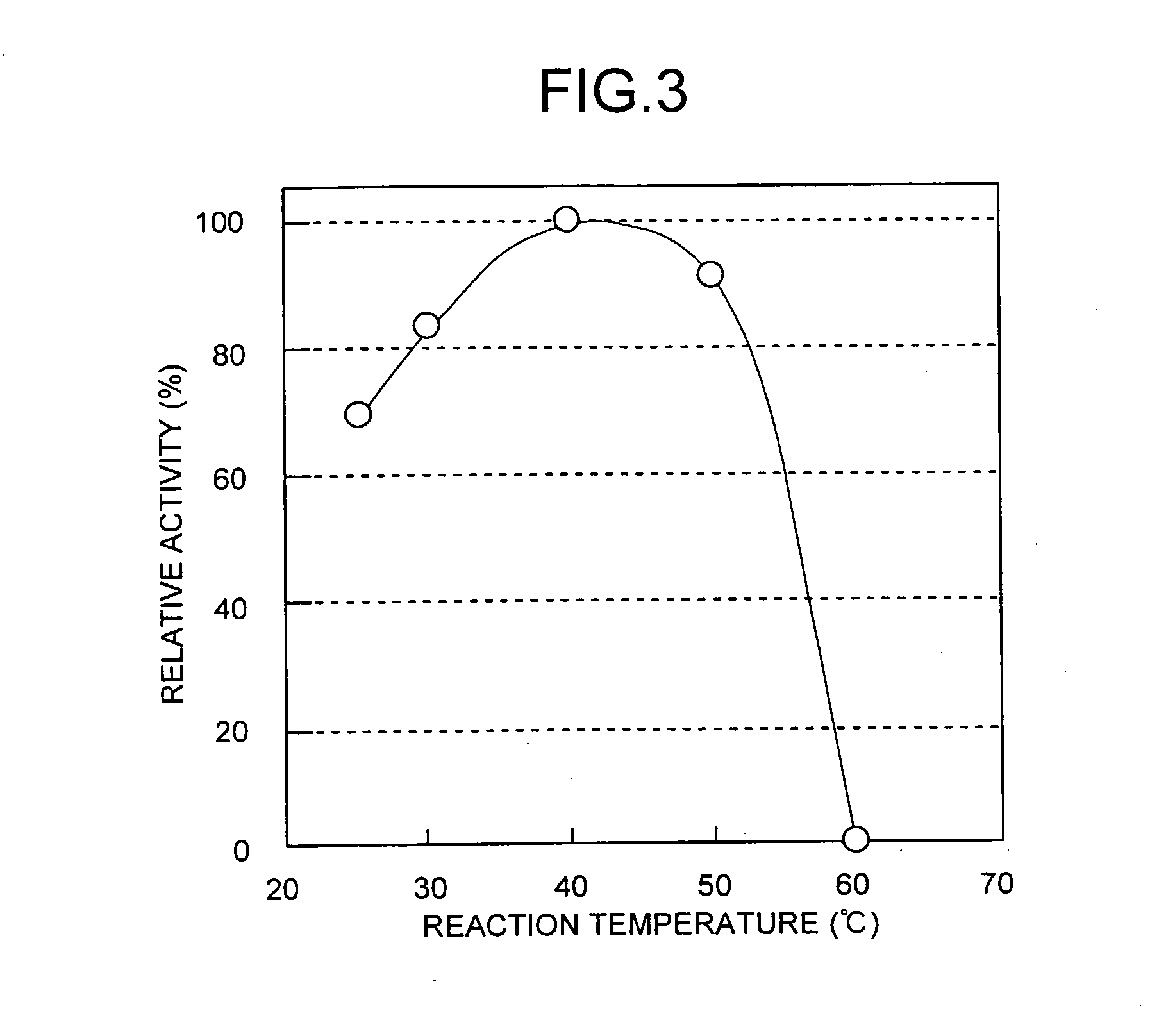
[0109] Measurement of enzyme titer was carried out in the manner described below. 200 micromoles (μmol) of Tris-HCl buffer (pH 9.0), 50 μmol of L-alanine amide hydrochloride and a suitable amount of enzyme liquid were added and mixed to bring to a final volume of 1 ml, and allowed to react at 30° C. for 60 minutes. Then, 4 ml of aqueous phosphate solution (pH 2.1) was added to stop the reaction. The L-alanine produced was quantified by high-performance liquid chromatography. The amount of enzyme that produced 1 μmol of L-alanine in 1 minute was defined as 1 unit of enzyme.
[0110] 8 liters (L) of Corynebacterium glutamicum strain ATCC 13286 was cultured in the same manner as Example 1 followed by collection of the microbial cells by centrifugal separation. The following procedure was carried out on ice or at 4° C. After washing the microbial cells with 50 mM potassium phosphate buffer (pH 7.0)...
example 3
Evaluation of Molecular Weight of L-Alanine Hydrolase
[0111] The equivalent of 0.5 microgram (μg) of the purified enzyme preparation obtained according to the method of Example 2 was applied to polyacrylamide electrophoresis. 0.3% (w / v) Tris, 1.44% (w / v) glycine and 0.1% (w / v) sodium lauryl sulfate were used for the electrophoresis buffer, concentration gradient gel having a gel concentration of 10 to 20% (Multigel 10 to 20, manufactured by Daiichi Pure Chemicals) was used for the polyacrylamide gel, and precision pre-stained standards (manufactured by Biorad) were used for the molecular weight markers. Following completion of electrophoresis, the gel was stained with Coomassie brilliant blue R-250, and a uniform band was detected at the location calculated to have a molecular weight of 42,000 to 46,000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com