Vaccine
a technology of nucleic acid constructs and vaccines, applied in the field of dna vaccines, can solve the problems of inability to encode, and the efforts so far have not been successful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
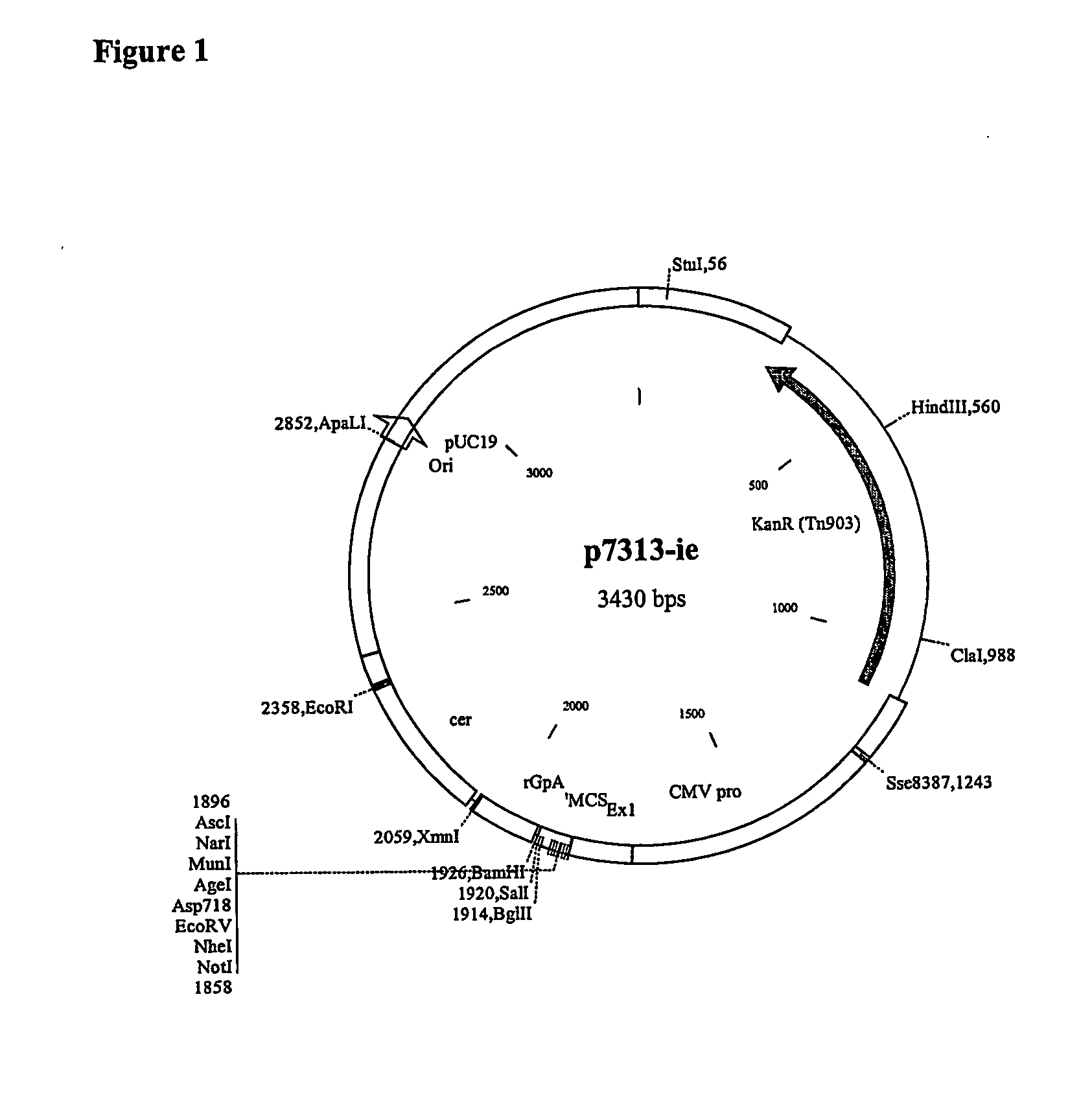
Plasmid Construction
[0163] 1.1 Construction of gp120 Containing Plasmid
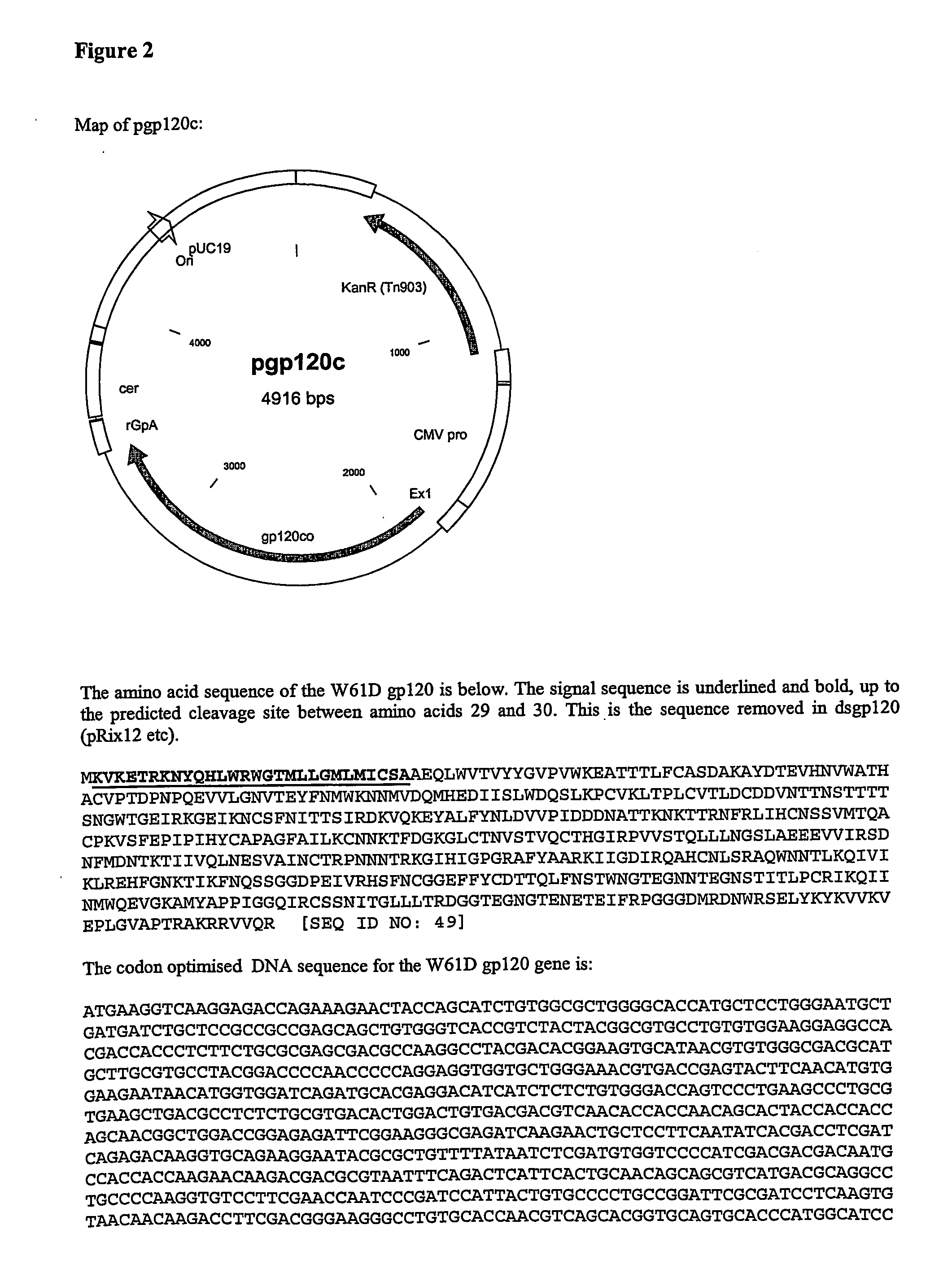
[0164] Recombinant gp120 glycoprotein described in the following examples is a synthetic form of the gp120 envelope protein of HIV-1 isolate W61D.
[0165] Codon Optimised (pgp120c):
[0166] The gene sequence was based on the gp120 sequence from the }[V-1 isolate W61D. This has a Codon Usage Coefficient of 0.297. Optimisation was performed using SynGene 2d, resulting in a CUC of 0.749 (Ertl, P F., Thomsen, L L. Technical issues in construction of nucleic acid vaccines. (2003) Methods 31(3); 199-206. SynGene uses a mathematical method for codon optimisation based on the relative frequencies of use. Briefly, codons are assigned value ranges according to their frequencies, so that more frequent codons have wider ranges, and placed in ascending frequency order. The value ranges are expressed as >=0.000, >=0.0??, >=0.??? And so on. A random number is generated between 0 and 0.99999. This is then used to select a codon,...
example 2
Modification of gp120 and Nef / Tat(mut) Expression Vectors
[0197] gp120 constructs were modified to reduce secretion of the protein.
[0198] Generation of Constructs:
[0199] gp120 Without a Secretion Signal (dsgp120, pRix 12—see FIGS. 2 and 6)
[0200] The gp120 gene was PCR amplified from pgp120c using the following primers:
5′120ds:5′GAATTCGCGGCCGCCATGGCCGAGCAGCTGTG[SEQ ID NO: 14]GGTCACCL01:5′GAATTCGGATCCTCATCTCTGCACGACGCGGC[SEQ ID NO: 15]GCTTGGCCCGGGTGGGGGCCACG
[0201] Fragments were amplified using PWO DNA polymerase (Roche) and the cycle:
[0202] The products were cut with NotI and BamHI and cloned into p7313-ie to give pRix12 (FIG. 6).
[0203] Results
[0204] In 293T cells the vector pRIX12, which lacks the secretion signal, makes a good amount of a 60 kDa non-glycosylated protein that is not secreted (FIGS. 29 and 30).
example 3
Construction of Vectors for Expression of gv120 and Nef / Tat(mut) From a Single Plasmid
[0205] Vector Construction:
[0206] The gp120 NefTat(m) constructs were generated by PCR stitching the gp120 and Nef / Tat(m) or trNef / Tat(m) orfs.
[0207] 5′ and 3′ Gp120, 5′ and 3′ Nef / Tat(m) and 5′trNef / Tat were amplified from pRix1. 3′trNef / Tat(m) was amplified from pNTm. The following primers were used:
3′120: (antisense to):GCCAAGCGCCGCGTCGTGCAGAGA[SEQ ID NO: 16]5′120 / NT:GCCAAGCGCCGCGTCGTGGAGAGAATGGGTGGCA[SEQ ID NO: 17]AGTGGTGAAAAAGT3′NT (antisense to):GGGGAGCCGACAGGCCCGAAGGAA[SEQ ID NO: 18]5′NT / 120:GGGGAGCCGACAGGCCCGAAGGAAATGAAGGTCA[SEQ ID NO: 19]AGGAGACCAGAAAG5′120 / trN:GCCAAGCGCCGCGTCGTGCAGAGAATGGTGGGTT[SEQ ID NO: 20]TTCCAGTCAC5′trNef:GAATTCGCGGCCGCCATGGTGGGTTTTCCAGTCA[SEQ ID NO: 21]CACCL01:GAATTCGGATCCTCATCTCTGCACGACGCGGCGC[SEQ ID NO: 22]TTGGCCCGGGTGGGGGCCACGL02:ACCACCTTGTACTTGTACAGCTCGCTCCGCCAGT[SEQ ID NO: 23]TATCCGTCATGTCGCCGCCGCCGGGC
[0208] Fragments were amplified using PWO DNA polymerase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com