Fluorescence polarization assay
a fluorescence polarization and assay technology, applied in the field of fluorescence polarization assay, can solve the problems of limited antibody-based molecular recognition molecules, and therefore useful in diagnostic assays, and the platform that has typically been used to identify and quantify aptamers bound to analytes is very limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0091] A. Methods for assessing binding of proteins to aptamer-coupled beads
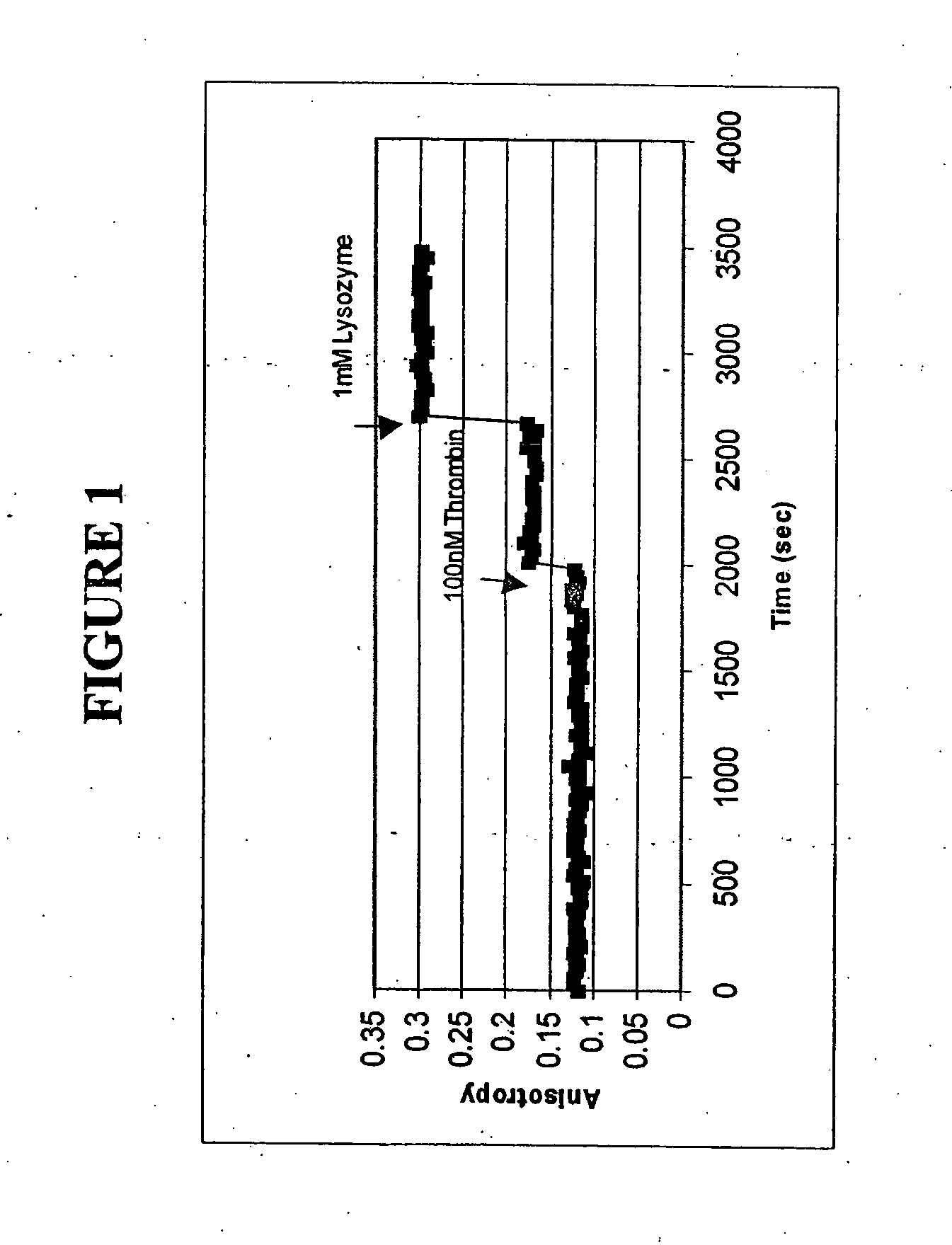
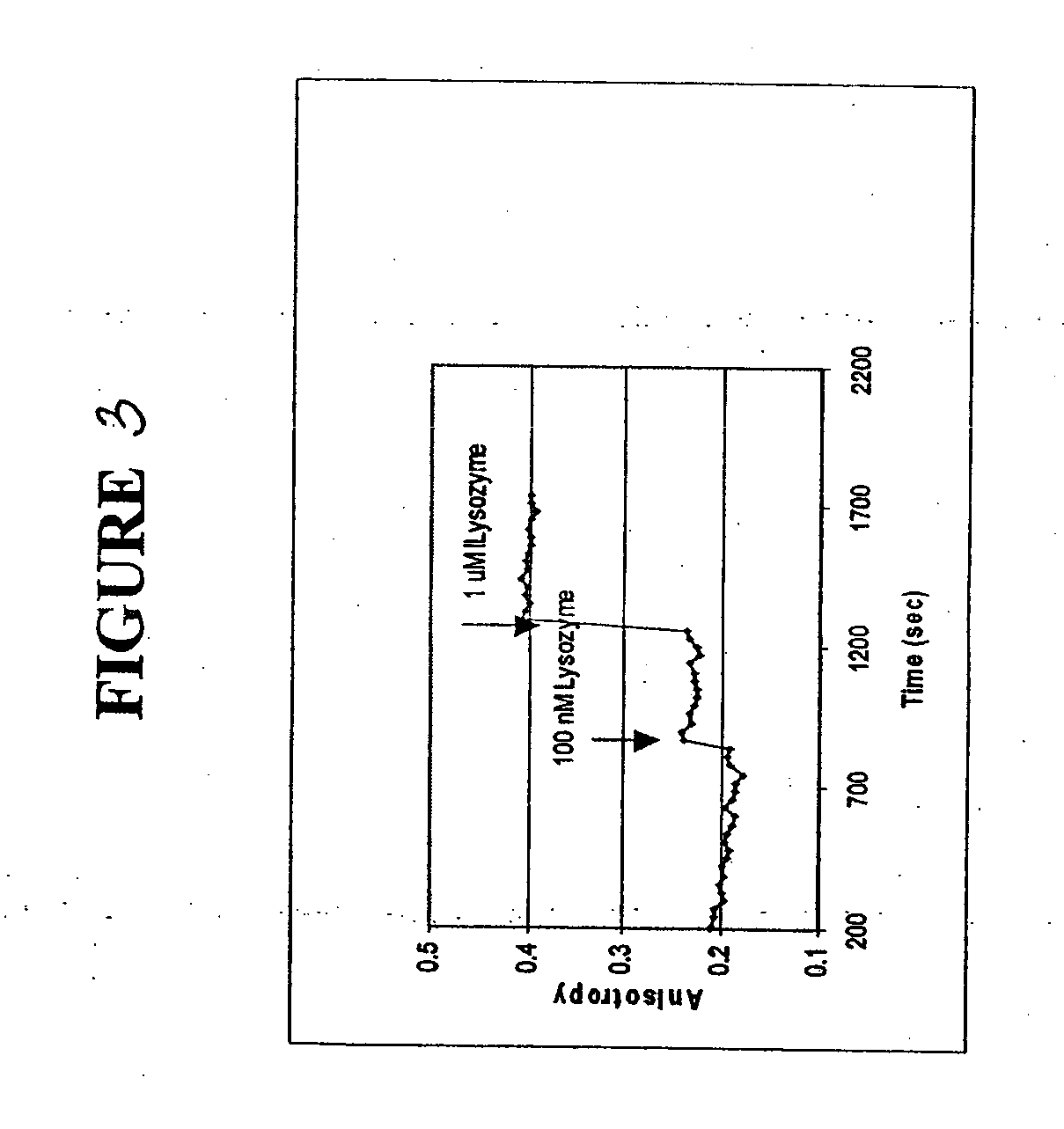
[0092] The polarization anisotropy of dye-labeled aptmers changes with time upon binding to a specific protein. Changes in fluorescence anisotropy of 3 aptamers bound to a solid support upon binding of specific proteins was measured.
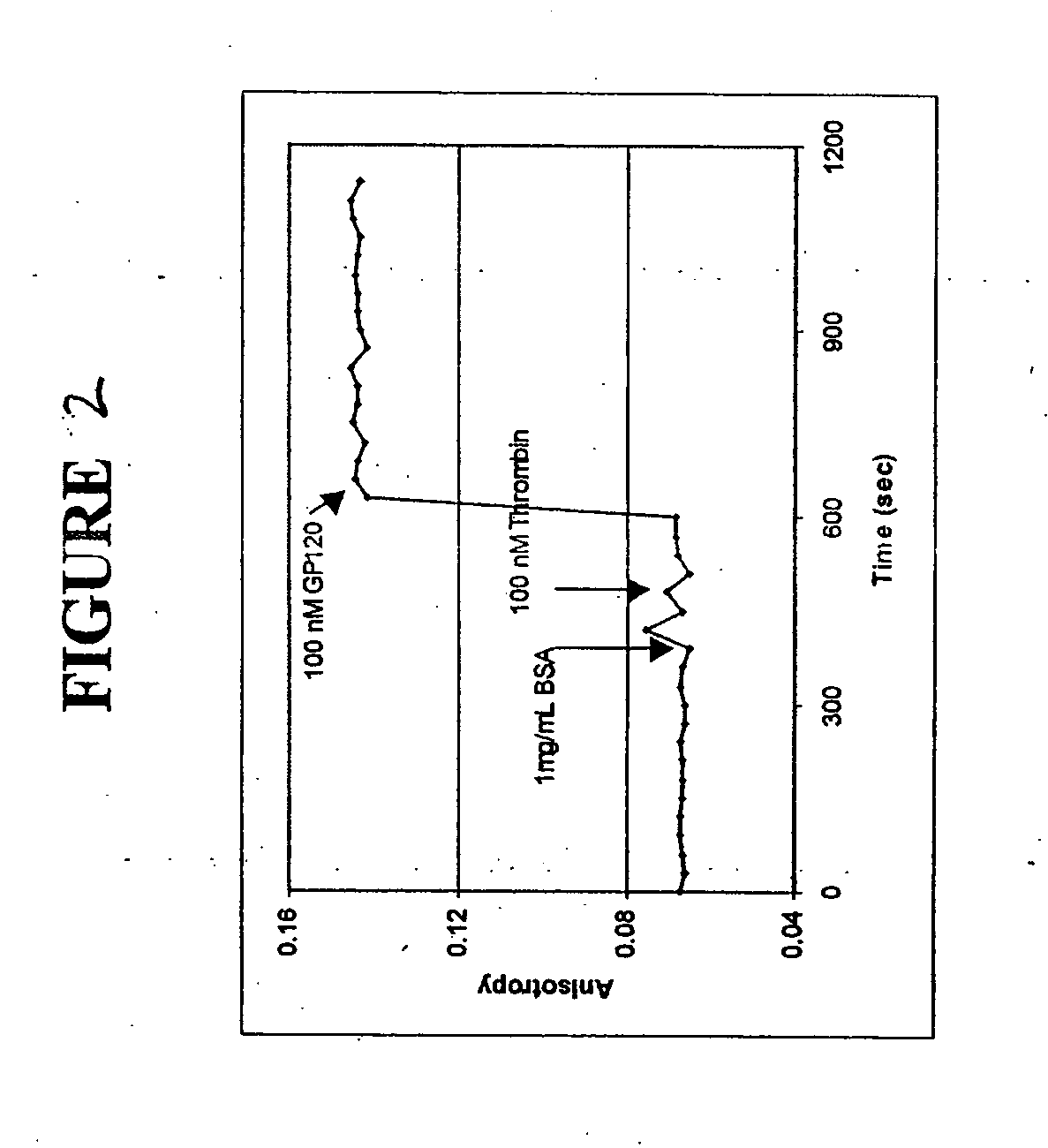
[0093] The three fluorophore labeled aptamers used in these experiments were anti-thrombin aptamer (FAM-labeled, a 15 mer), which is specific for thrombin from human plasma, 518-aptamer (FAM-labeled, a 60 mer), which is specific for GP120MN, an HIV-1 protein and 650-aptamer (FAM-labeled, a 60 mer), which is specific for recombinant human FGF basic protein.
[0094] 1. Anti-Thrombin Aptamer for Thrombin
[0095] Binding of thrombin to FAM-labeled anti-thrombin aptamers was measured. 5 micron silica particles were purchased from Bangs Laboratories (Ohio, USA) and pretreated with 1N NaCI solution at 100° C. for 1 hour. The silica particles were then treated for 30 minutes with 3-amino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com