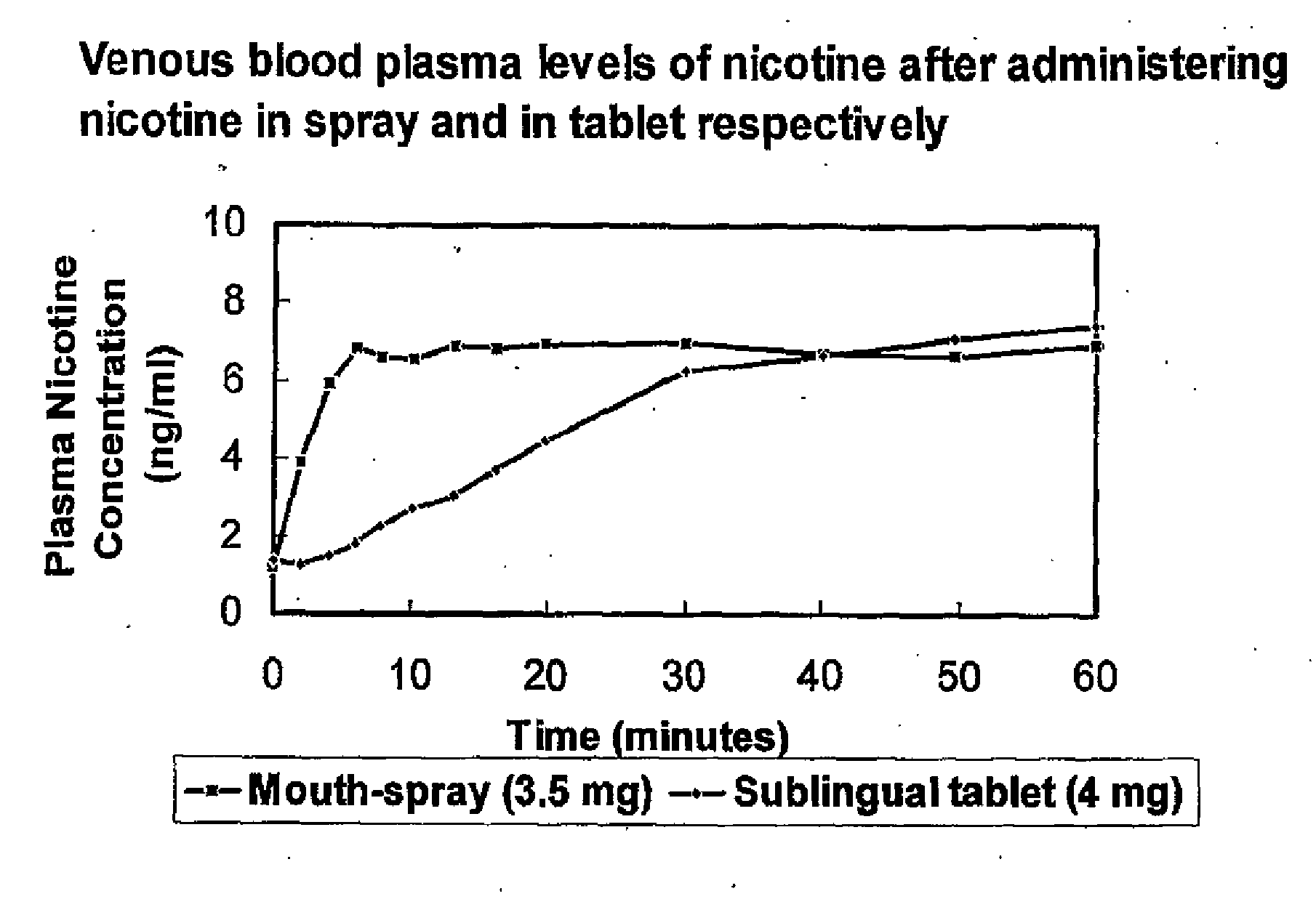
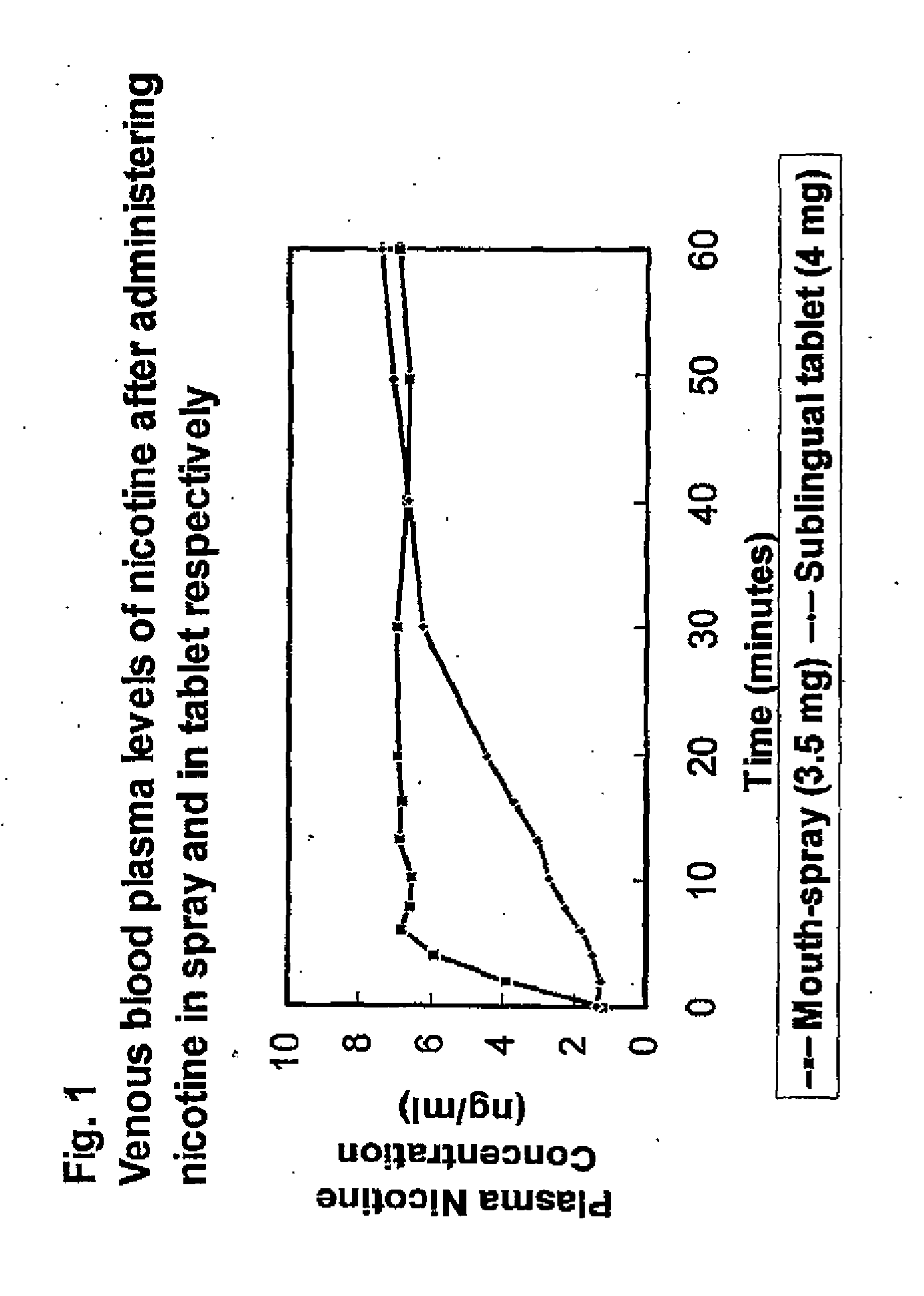
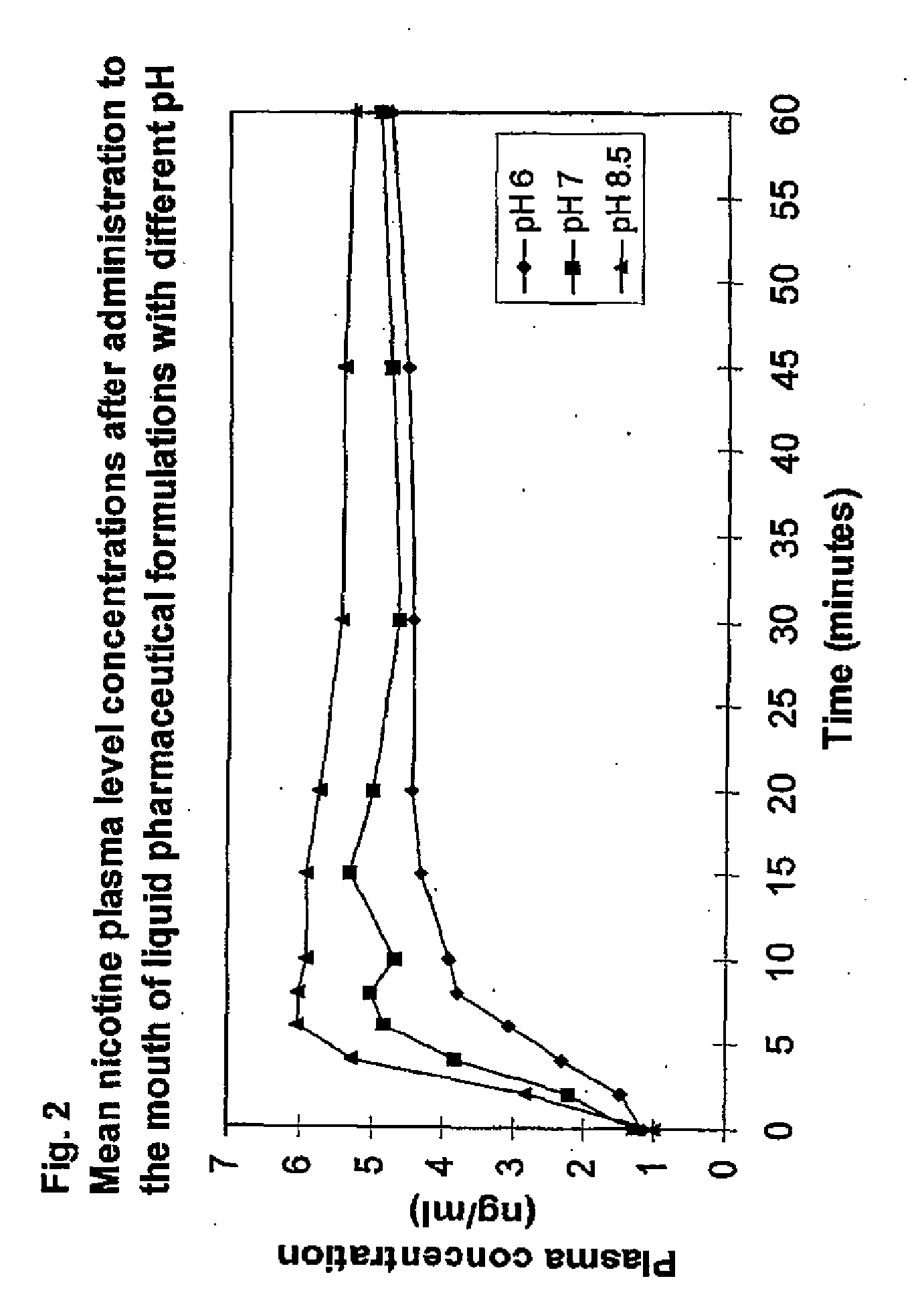
Formulation and Use and Manufacture Thereof
a technology of liquid pharmaceuticals and pharmaceutical formulations, applied in the direction of medical preparations, pharmaceutical delivery mechanisms, tobacco, etc., can solve the problems of not satisfying the craving, not providing for a sufficiently rapid uptake of nicotine without, and not satisfactorily fulfilling the hitherto known means and methods, etc., to achieve rapid and/or sustained and/or complete reduction of the urge to smoke or use tobacco.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0148] Manufacturing of a 1000 ml formulation with 10 mg nicotine / ml and around pH 8.5.
[0149] Mixture 1
[0150] To a beaker containing 800 ml water of 90° C. was added 0.7 g methyl para-hydroxybenzoate, acting as preservative, and 0.3 g propyl para-hydroxybenzoate, acting as preservative. The additives were dissolved during stirring for about 10 minutes. Then was added 10.45 g sodium dihydrogen phosphate, acting as buffering agent, and 0.5 g EDTA, acting as chelating agent, to the solution, which was stirred for about 5 minutes. Then the solution was cooled to 30° C. during stirring.
[0151] Mixture 2
[0152] To a beaker containing 15.9 g ethanol of room temperature, acting as solvent, was added 0.045 g peppermint oil, acting as flavoring agent. The liquid was mixed for 2 minutes.
[0153] Final Mixture
[0154] Mixture 2 was added during stirring to a beaker containing 150 ml water. Gently 10 g nicotine (base) was added to the beaker. Then Mixture 1 was added to the beaker and stirred fo...
example 2
[0155] Manufacturing of a 1000 ml formulation with 10 mg nicotine / ml and around pH 7.0.
[0156] This Example 2 differs from Example 1 only for pH. The formulation according to Example 2 contains a non-alkalizing buffering agent. This formulation was for use as a comparison in FIG. 2.
[0157] Mixture 1
[0158] To a beaker containing 800 ml water of 90° C. was added 0.7 g methyl para-hydroxybenzoate, acting as preservative, and 0.3 g propyl para-hydroxybenzoate, acting as preservative. The additives were dissolved during stirring for about 10 minutes. Then was added 10.45 g sodium dihydrogen phosphate, acting as buffering agent, and 0.5 g EDTA, acting as chelating agent, to the solution, which was stirred for about 5 minutes. Then the solution was cooled to 30° C. during stirring.
[0159] Mixture 2
[0160] To a beaker containing 15.9 g ethanol of room temperature, acting as solvent, was added 0.045 g peppermint oil, acting as flavoring agent. The liquid was mixed for 2 minutes.
[0161] Fina...
example 3
[0163] Manufacturing of a 1000 ml formulation with 10 mg nicotine / ml and around pH 6.0.
[0164] This Example 3 differs from Example 1 only for pH. The formulation according to Example 3 contains a non-alkalizing buffering agent. This formulation was for use as a comparison in FIG. 2.
[0165] Mixture 1
[0166] To a beaker containing 800 ml water of 90° C. was added 0.7 g methyl para-hydroxybenzoate, acting as preservative, and 0.3 g propyl para-hydroxybenzoate, acting as preservative. The additives were dissolved during stirring for about 10 minutes. Then was added 10.45 g sodium dihydrogen phosphate, acting as buffering agent, and 0.5 g EDTA, acting as chelating agent, to the solution, which was stirred for about 5 minutes. Then the solution was cooled to 30° C. during stirring.
[0167] Mixture 2
[0168] To a beaker containing 15.9 g ethanol of room temperature, acting as solvent, was added 0.045 g peppermint oil, acting as flavoring agent. The liquid was mixed for 2 minutes.
[0169] Fina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com