Cannabinoid derivatives, methods of making, and use thereof
a technology of cannabinoid derivatives and derivatives, applied in the field of cannabinoid derivatives, can solve the problems of limiting the ability to predict the preferred side chain geometry and the lbp steric requirements of the cb receptor, and achieve the effect of improving the affinity of the cb-1 and/or cb-2 receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Binding Assays
[0152] Cell membranes from HEK293 cells transfected with the human CB1 cannabinoid receptor and membranes from CHO-K1 cells transfected with the human CB2 cannabinoid receptor were used in the receptor binding assays. Displacement of [3H]CP 55,940 from the CB1 and CB2 receptor preparations by increasing concentrations of the Δ8-THC analogs 23-29 and Δ8-THC were used to determine the binding affinities of the conformationally biased probes (see Table 1 below). The Ki values for Δ8-THC at the hCB1 and hCB2 receptor were 28.5 nM and 25.0 nM, respectively (affinity ratio CB1 / CB2=1.14), compared to a reported value of 47.6 nM for the rCB1 and 39.3 for the mCB2 (affinity ratio CB1 / CB2=1.21) (Busch-Petersen, J. et al., J. Med. Chem. 39:3790 (1996), which is hereby incorporated by reference in its entirety). The LBP probes exhibited a 3 to 143 fold enhancement in binding affinity to the receptor subtypes relative to Δ8-THC. The gem-dimethyl analogs 27-29 and the pentyl dithiol...
example 3
olecular Modeling Studies
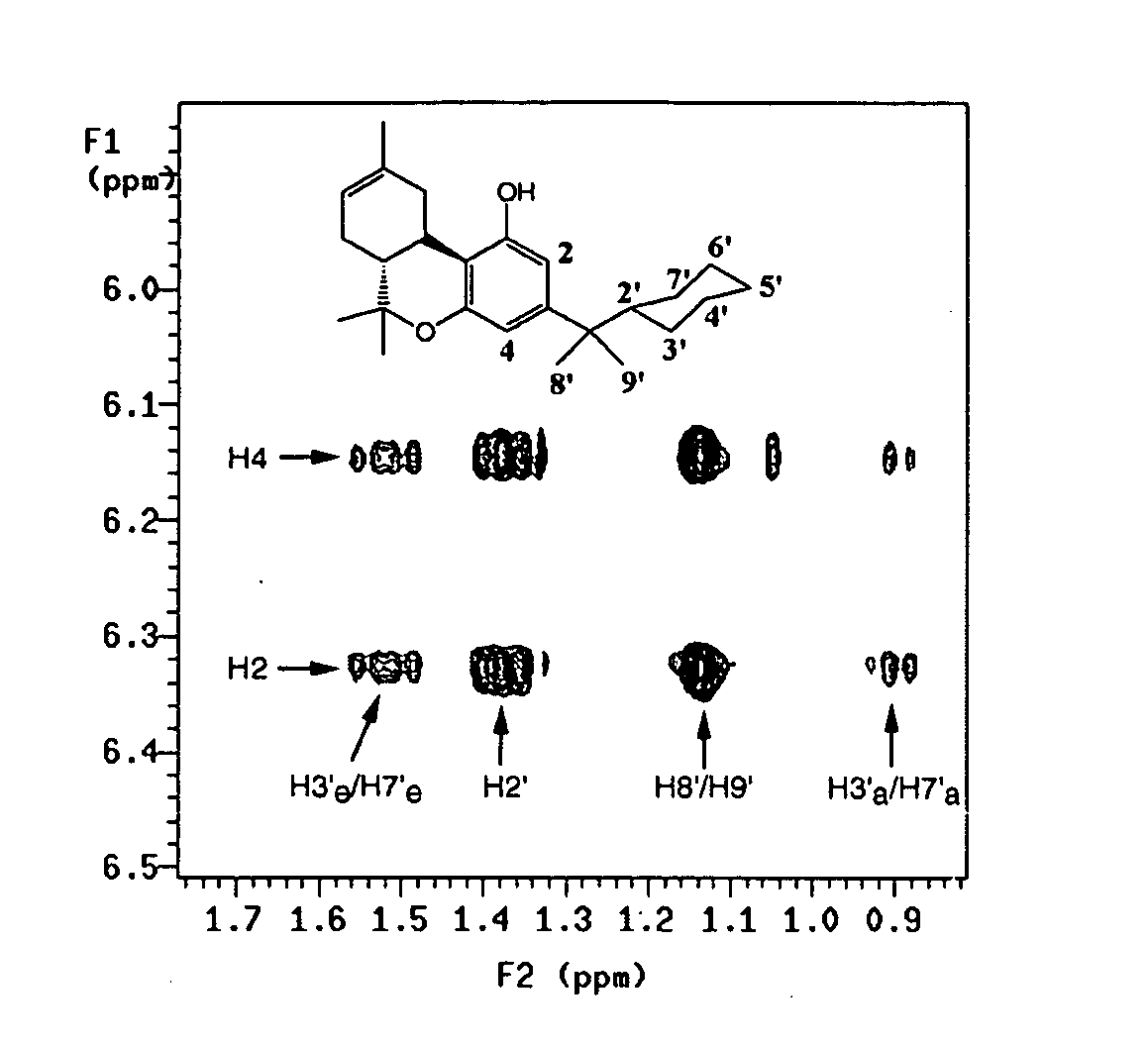
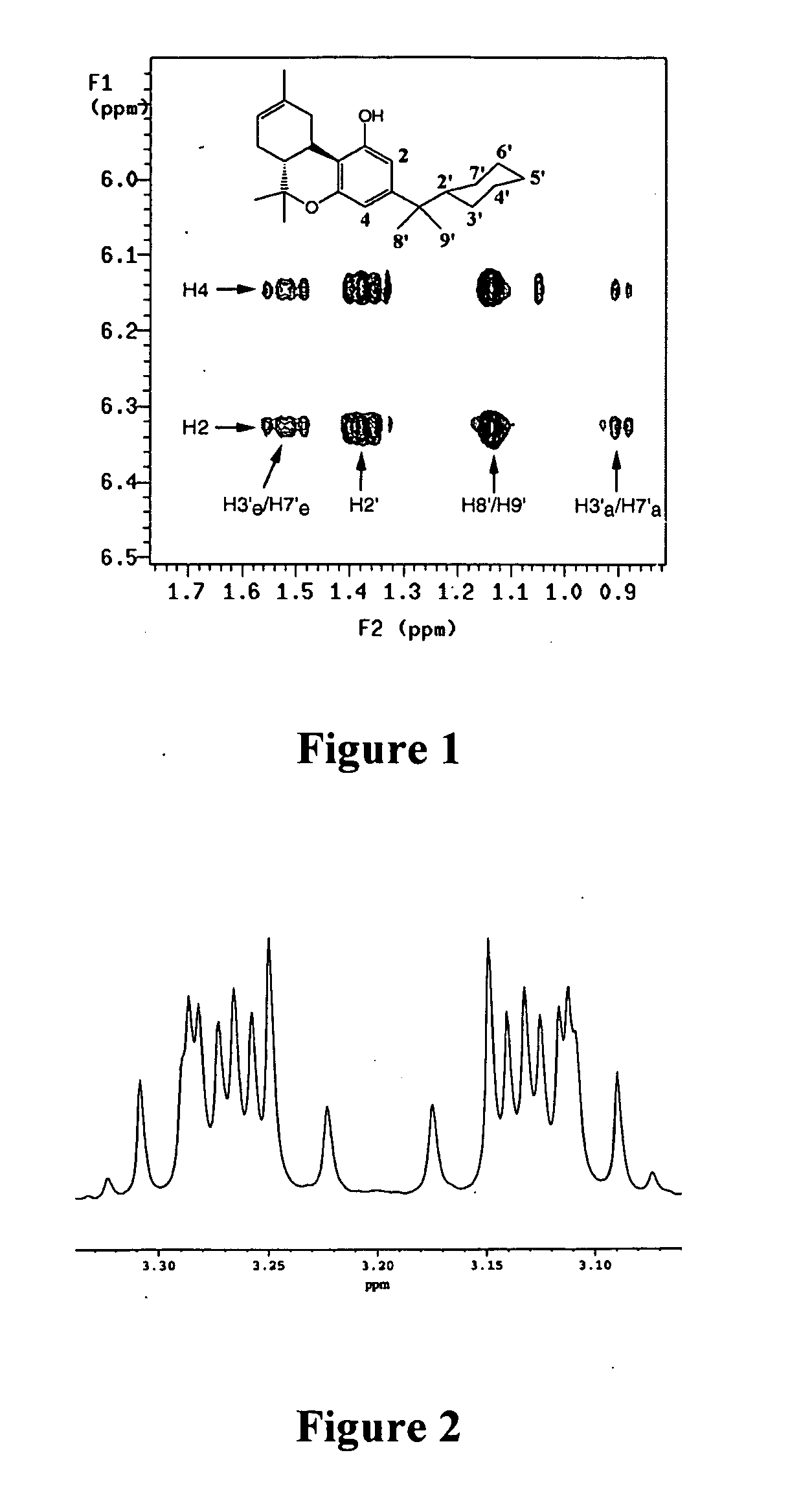
[0154] The binding affinities of the dimethyl-cycloalkyl analogs for both the CB1 and CB2 receptors are comparable to that of the highly potent 1′,1′-dimethylheptyl THC (DMHT, Ki=0.77 nM) analog. Molecular modeling analysis of these compounds shows that the linear dimension of DMHT is 7.72 Å compared to 4.55 Å for 28, which is the cyclic carbon equivalent of DMHT. The difference of 3.17 Å in the linear length of the side chain suggested that there is a region in the LBP that accommodates the C3 substituent which can be characterized as a hydrophobic ellipsoid. It remains unclear if this is the same pocket that accommodates the side chain of linear analogs; however, the potential existence of an ellipsoid pocket made it important to characterize the relative geometry of the cyclic side chain with respect to the tricyclic ring system. One facet of this effort utilized 1D and 2D high field NMR spectroscopy to assess the relative side chain geometries; furthermo...
example 4
echanical Calculations
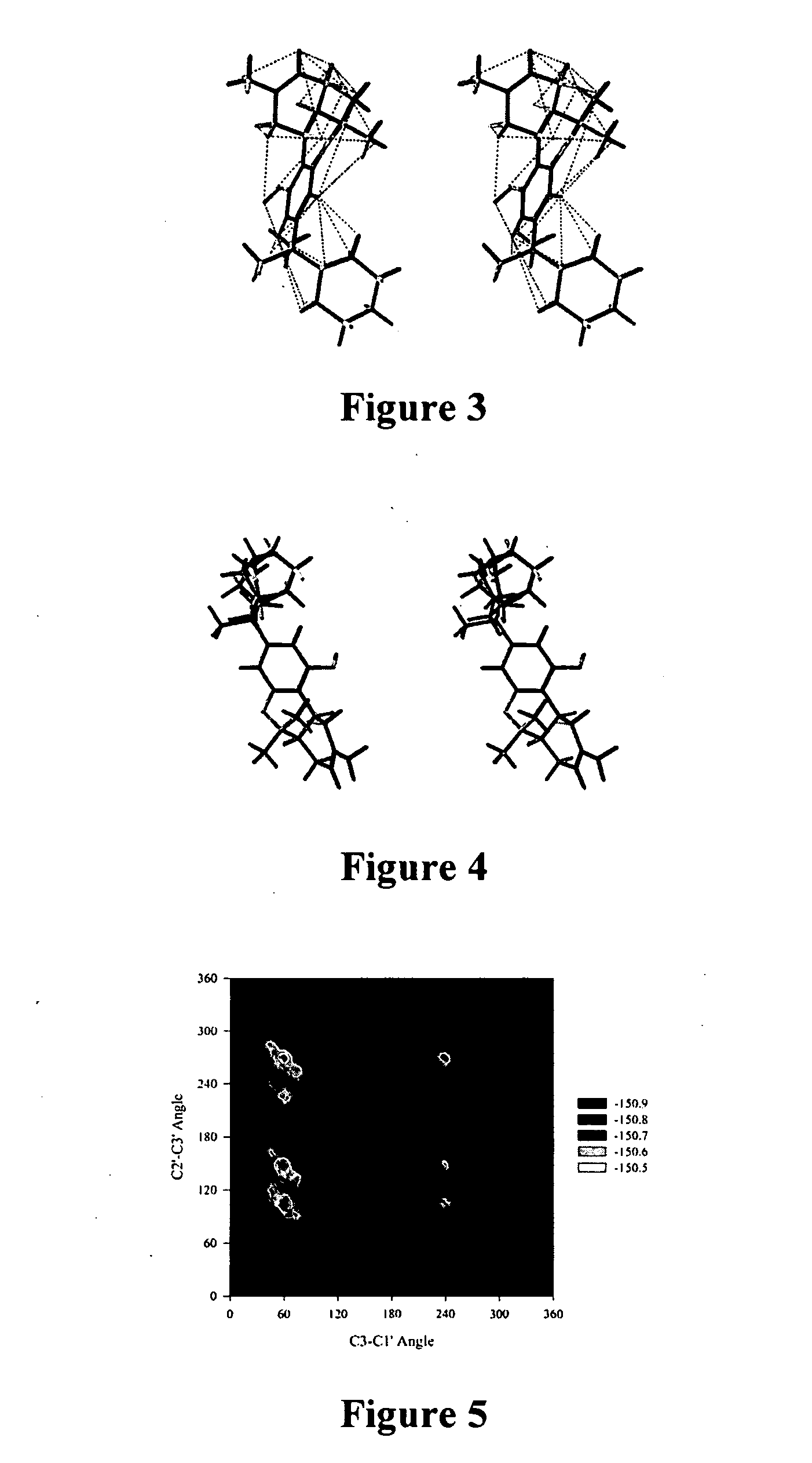
[0157] It seemed reasonable to assume that the gem-dimethyl would adopt a conformer that would maximize the interaction between the conformationally biased side chain and the aromatic ring. However, the conformation of the cyclohexyl analog 28 predicted by NMR and molecular dynamics could not be explained based on molecular mechanics calculations, i.e. electrostatic and steric analysis. To address this issue, semi-empirical and density functional theory (DFT) calculations have been employed to critically evaluate the potential conformers and determine the importance of electronic contributions in the experimental results.
[0158] Geometry optimizations were performed using both the AM1 and PM3 semi-empirical parameterizations with the GAMESS computational chemistry package (Schmidt, M. W. et al., J. Comput. Chem. 14:1347 (1993), which is hereby incorporated by reference in its entirety). The stationary points were confirmed by calculating the Hessian at the opti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical shifts | aaaaa | aaaaa |
| chemical shifts | aaaaa | aaaaa |
| dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com