Animal model for prostatic stromal hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Animal Model having Prostate-Like Stroma-Enlarged Tissue
(1) Removal of Rat Fetal Urogenital Sinus
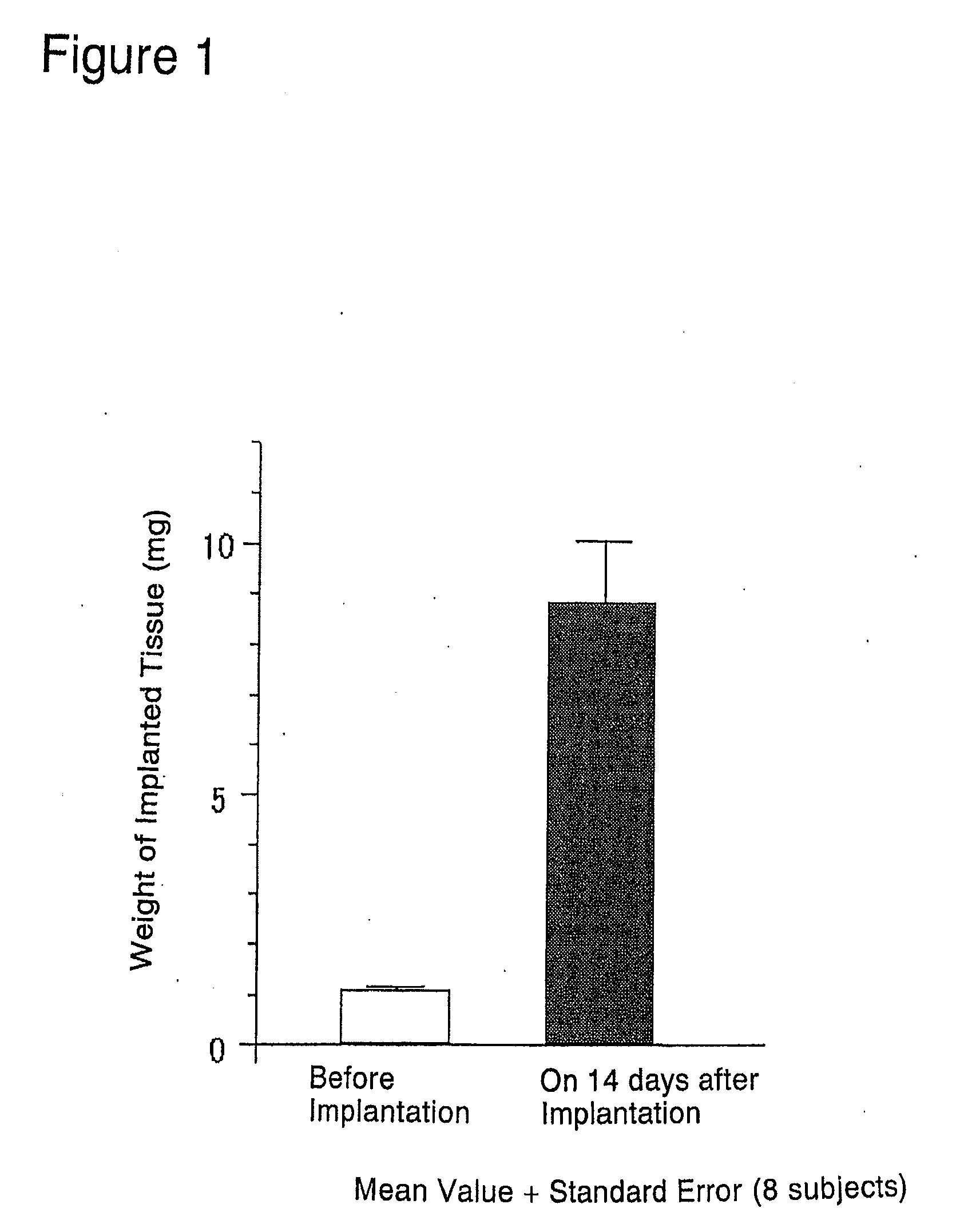
[0171]Under ether anesthesia, fetuses were removed from female SD rats that were 21 days pregnant. From the removed male fetuses, urogenital sinuses were removed also under ether anesthesia while observing through a stereoscopic microscope. The urogenital sinuses were stored in a sterilized culture medium until implantation.
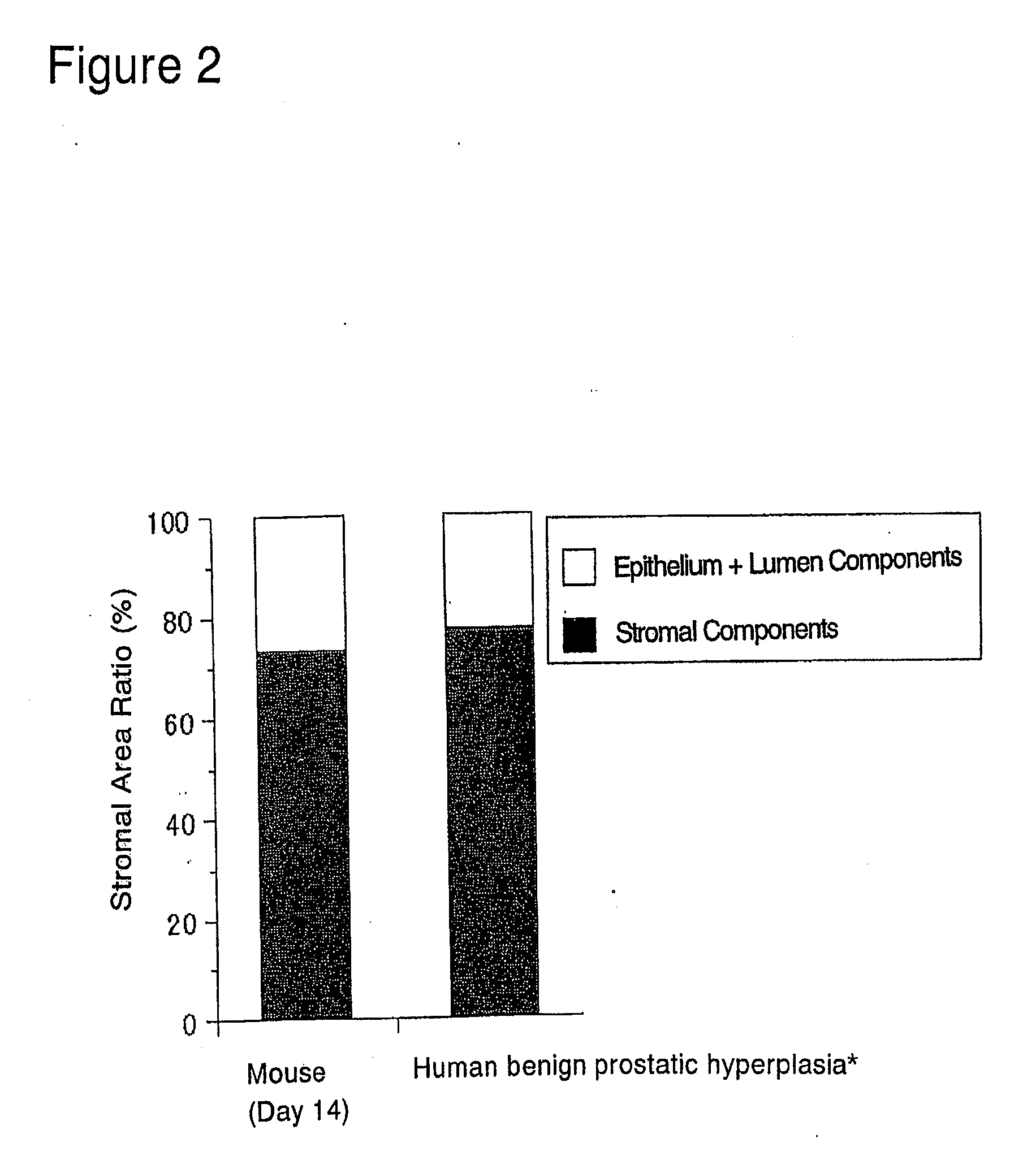
[0172]The abdominal skin adjacent the left femoral region of 6-week old BALB / c male nude mice was cut open about 2 to 3 mm under ether anesthesia. A single rat fetal urogenital sinus obtained in (1) above was subcutaneously implanted after incision using a forceps and the incision was sutured. Thereafter, these nude mice were reared in clean racks (Rat Bracket Cages, 3 to 4 mice / cage) for 2 weeks under the following conditions:[0173]Temperature: 20 to 26° C.[0174]Humidity: 30 to 70%[0175]Lighting hours: 12-hour light-dark cycle;...
example 2
Production of Animal Model for Prostatic Stromal Hyperplasia
(1) Removal of Rat Fetal Urogenital Sinus
[0186]Under ether anesthesia, fetuses were removed from female SD rats that were 20 days pregnant. From the removed fetuses, the urogenital sinuses were removed under ether anesthesia while observing through a stereoscopic microscope. The urogenital sinuses were stored in a sterilized culture medium until implantation.
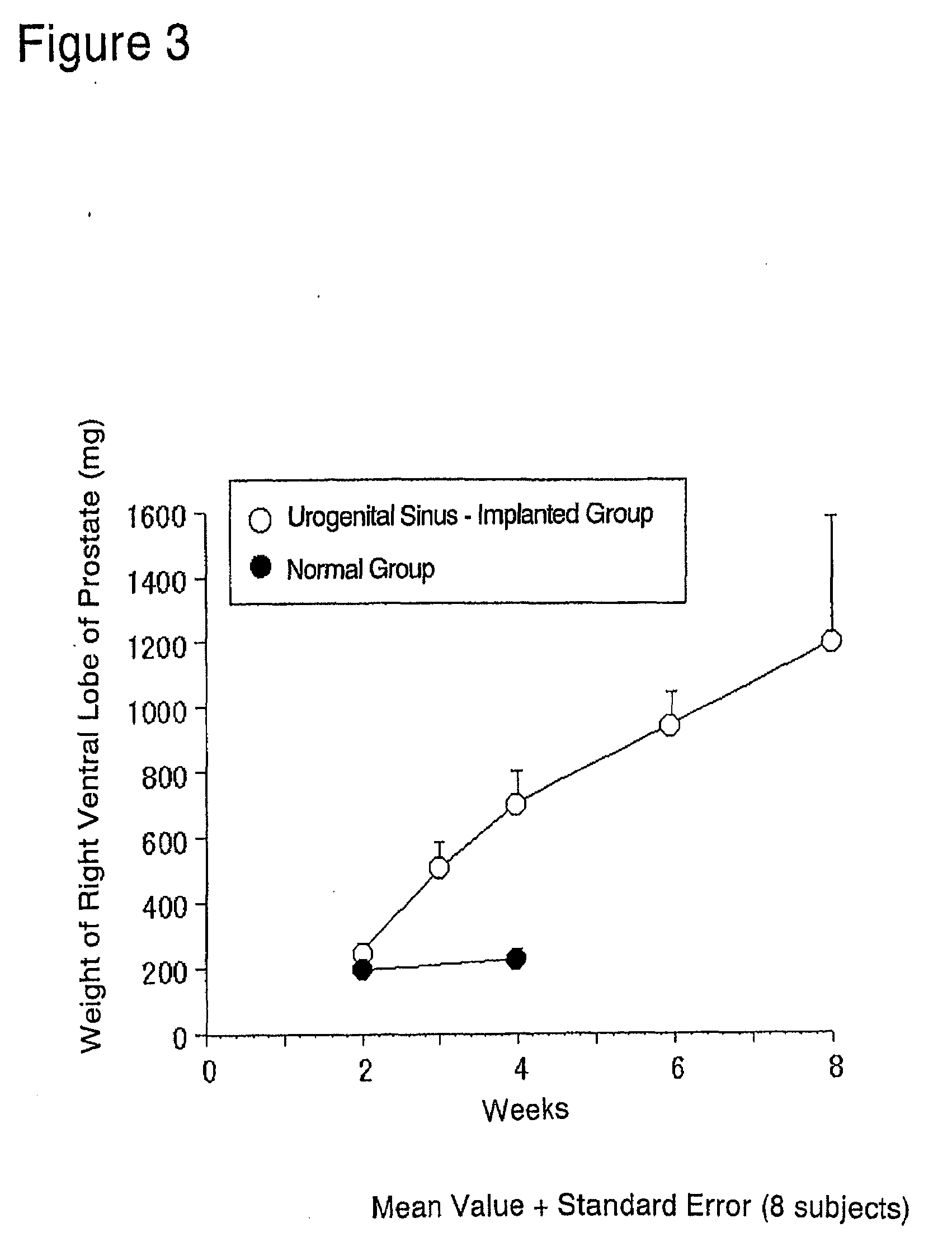
[0187]The prostate glands were exposed by midline incision using 6-week old male SD rats under ether anesthesia. The capsule of prostate right ventral lobe was slightly cut open while observing through a stereoscopic microscope. Two of the fetal urogenital sinuses obtained in (1) above were implanted using a forceps beneath the capsule, and the incision in the capsule was sutured. The cut abdominal skin then closed and the rats were reared for 2 to 8 weeks in normal rearing cages (Rat Bracket Cages, 3 to 4 rats / cage) under the following conditi...
example 3
Production of Animal Model for Prostatic Stromal Hyperplasia
(1) Removal of Rat Fetal Urogenital Sinus
[0201]Urogenital sinuses were removed from the fetuses of female SD rats (donor animal) that were 20 days pregnant in the same manner as in Example 2 (1). The urogenital sinuses were stored in a sterilized culture medium until implantation.
[0202]In the same manner as in Example 2 (2), a single rat fetal urogenital sinus obtained in (1) above was implanted beneath the capsule of prostate right ventral lobe of each 6-week old male SD rat (recipient animal) (n=18×2), and the prostatic capsule was sutured. The implanted rats were reared in normal rearing cages for 3 to 6 weeks thereafter.
(3) Weighing of Implanted Tissue (Rat Fetal Urogenital Sinus-Derived Tissue)
[0203]The implanted tissues (rat fetal urogenital sinus-derived tissues) were removed from the above-prepared rats under ether anesthesia on 3 weeks (n=18) and 6 weeks (n=18) after implantation, and were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com