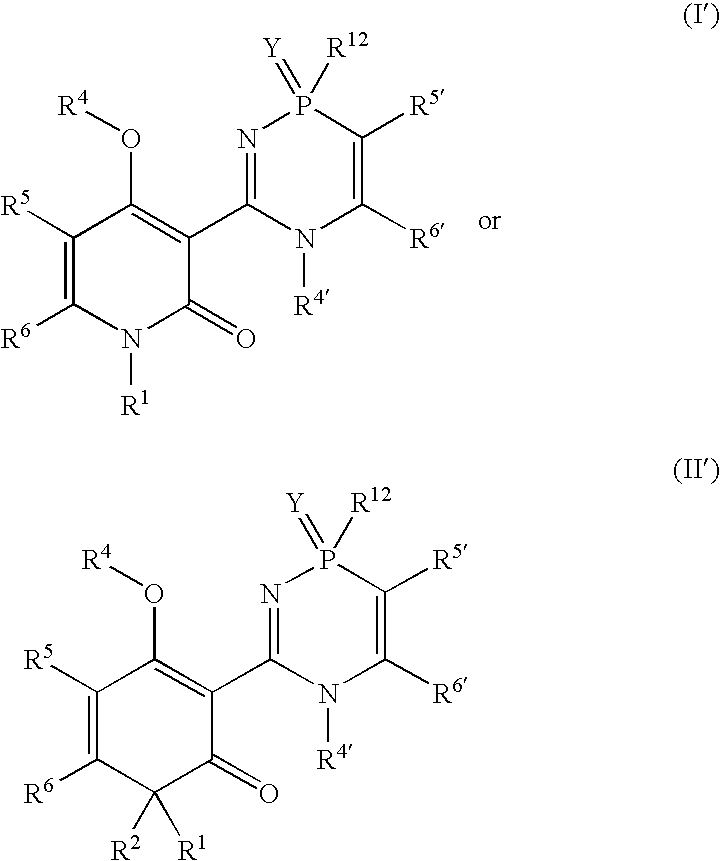
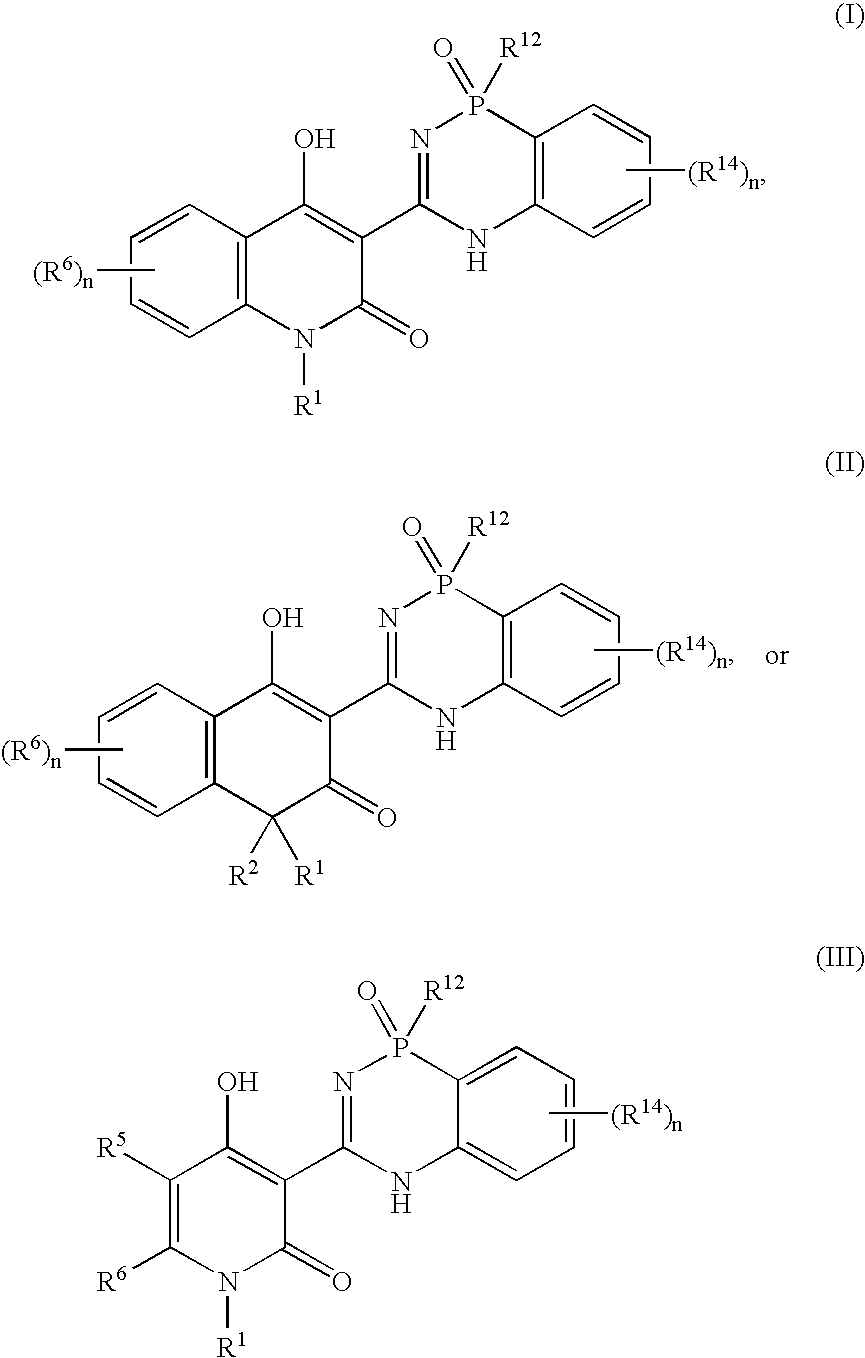
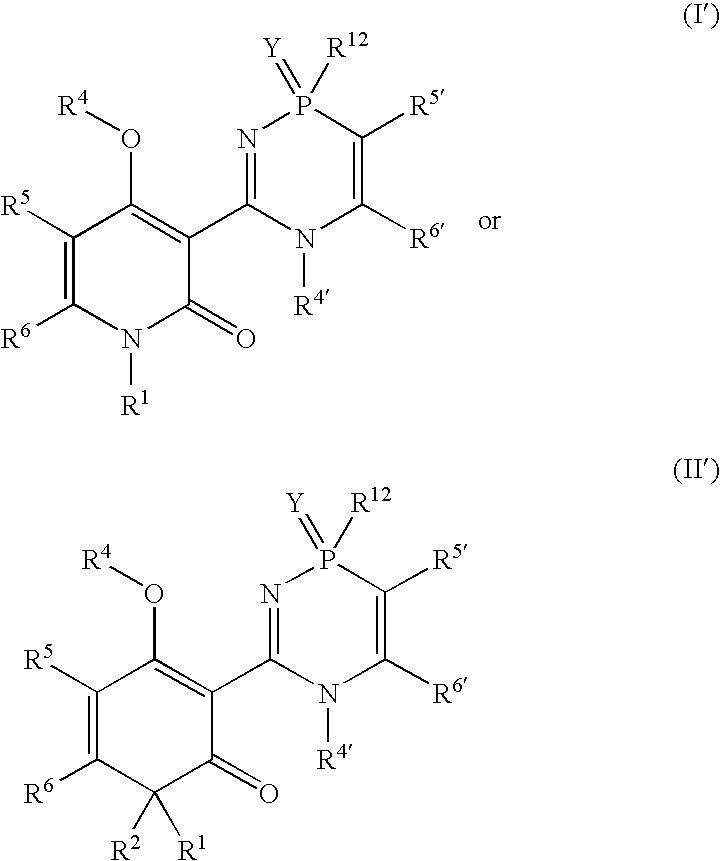
Phosphadiazine hcv polymerase inhibitors i and ii
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(2-cyclopropylethyl)-(1-ethoxy-1-oxo-4H-benzo[1,2,4]phosphadiazine 3-yl)-6-fluoro-dihydro-4-hydroxy-2-oxoquinoline
[0502]
[0503]Intermediate 5 (90 mg, 0.18 mmol) was dissolved in dimethylacetamide (1 ml), heated under microwaves radiations to 200° C. for 4.5 hours. After cooling, the reaction mixture was added dropwise to 25 ml buffer pH 7 (0.1M). The product was extracted in ethyl acetate, after evaporation of the organic layer, the residue was purified by silica gel chromatography (petroleum ether / ethyl acetate) to give Example 1 (24.3 mg, 40%), which was a white powder. Example 1 was characterized by the following spectroscopic data: 1H NMR (d6-DMSO, 400 MHz) δ (ppm) 0.07-0.10 (m, 2H), 0.39-0.44 (m, 2H), 0.76-0.87 (m, 1H), 1.21 (t, J=6.99 Hz, 3H), 1.56 (q, J=7.36 Hz, 2H), 3.99-4.05 (m, 2H), 4.33-4.36 (m, 2H), 7.40-8.08 (m, 7H); 19F NMR (d6-DMSO, 376 MHz) δ (ppm) −120.47 (s, 1F); 31P NMR (d6-DMSO, 162 MHz) δ (ppm) −0.04 (s, 1P); and MS (ESI, EI+) m / z=456 (MH+).
example 2
1-(2-cyclopropylethyl)-(1-ethoxy-1-oxo-4H-benzo[1,2,4]phosphadiazine 3-yl)-6-fluoro-dihydro-4-hydroxy-2-oxoquinoline sodium salt
[0504]
[0505]Example 1 (10 mg, 0.0018 mmol), NaOH 0.1M (187 μl, 1 eq) were stirred in dioxane (2 ml) and water (2 ml) at 40° C. for 10 min and freeze dried to give Example 2, which was a white powder. Example 2 was characterized by the following spectroscopic data: 1H NMR (d6-DMSO, 400 MHz) δ (ppm) 0.06-0.15 (m, 2H), 0.40-0.46 (m, 2H), 0.73-0.82 (m, 1H), 1.08 (t, J=7.01 Hz, 3H), 1.43-1.49 (m, 2H), 3.45-3.56 (m, 1H), 3.63-3.73 (m, 1H), 4.01-4.22 (m, 2H), 7.10 (t, J=7.4 Hz, 1H), 7.16 (t, J=7.4 Hz, 1H), 7.27-7.35 (m, 2H), 7.46-7.55 (m, 2H), 7.76 (dd, J=9.54 Hz and J=2.72 Hz, 1H), 14.9 (s, 1H); 19F NMR (d6-DMSO, 376 MHz) δ (ppm) −124.59 (s, 1F); 31P NMR (d6-DMSO, 162 MHz) δ (ppm) 3.24 (s, 1P); and MS (ESI, EI+) m / z=456 (MH+).
example 3
1-(2-cyclopropylethyl)-(1-hydroxy-1-oxo-4H-benzo[1,2,4]phosphadiazine 3-yl)-6-fluoro-dihydro-4-hydroxy-2-oxoquinoline
[0506]
[0507]The chromatography column of example 2 was flushed with methanol, the methanol was concentrated and the residue was precipitated in a minimum of methanol, filtered, washed with cold methanol and dried under reduced pressure, to give Example 3 (185 mg), which was a white powder. Example 3 was characterized by the following spectroscopic data: 1H NMR (d6-DMSO, 400 MHz) δ (ppm) 0.06-0.09 (m, 2H), 0.38-0.43 (m, 2H), 0.75-0.82 (m, 1H), 1.54 (q, J=7.22 Hz, 2H), 4.28 (t, J=7.22 Hz, 2H), 7.39-7.47 (m, 2H), 7.54-7.64 (m, 2H), 7.68 (t, J=7.72 Hz, 1H), 7.74-7.84 (m, 2H); 19F NMR (d6-DMSO, 376 MHz) δ (ppm) −120.77 (s, 1F); 31P NMR (d6-DMSO, 162 MHz) δ (ppm) −5.51 (s, 1P); and MS (ESI, EI+) m / z=428 (MH+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com