Stents with polymer-free coatings for delivering a therapeutic agent
a technology of stents and coatings, applied in the field of stents with polymer-free coatings for delivering a therapeutic agent, can solve the problems of coatings containing a coatings containing such a type of therapeutic agent without a polymer being generally ineffective in delivering the therapeutic agent, and presenting certain other limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
5.1 The Medical Device
[0027]The medical devices described herein generally include a substrate having at least one surface. For instance, in the case where the medical device is an intravascular stent, the substrate is the stent sidewall structure and the surface is the abluminal surface of the stent. A cavity is disposed in the substrate and a pellet comprising a non-polymeric material having a plurality of pores therein is disposed in the cavity. A therapeutic agent is disposed in at least some of the pores for delivery to a patient.
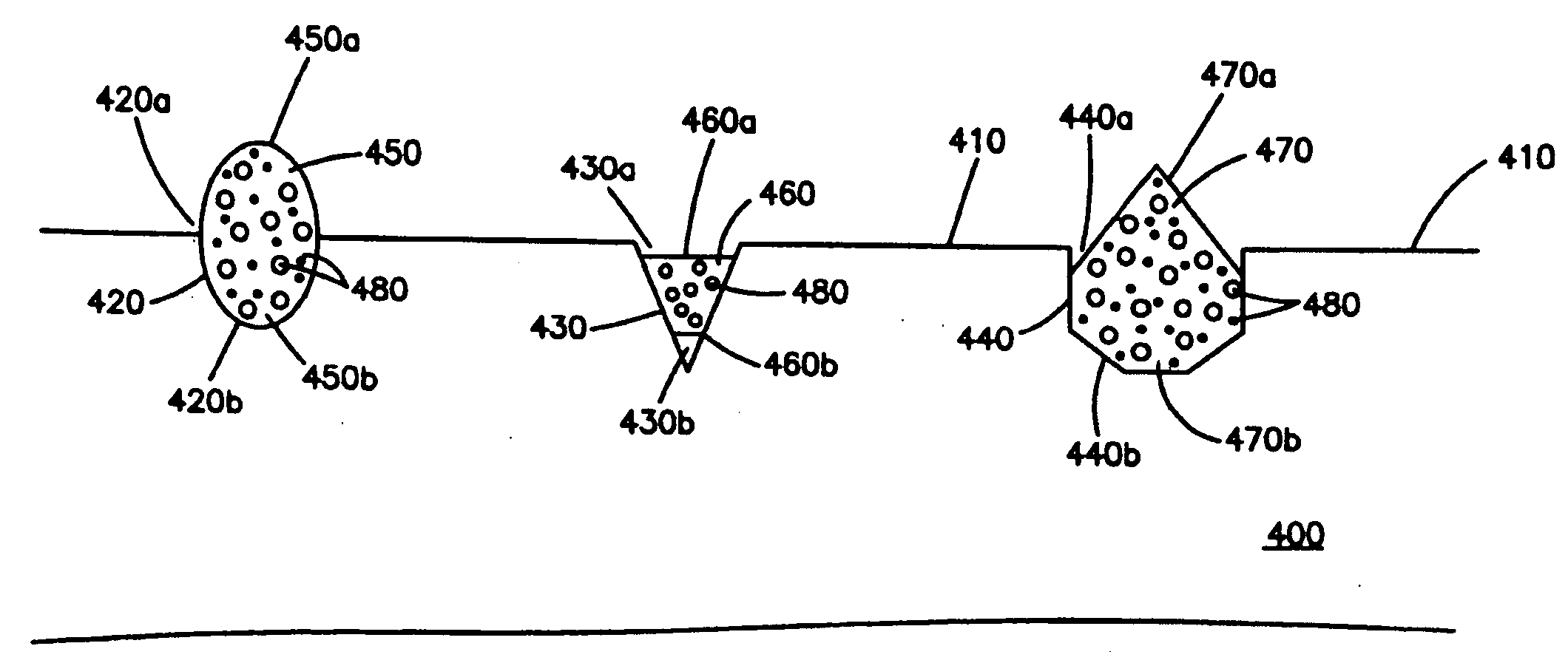
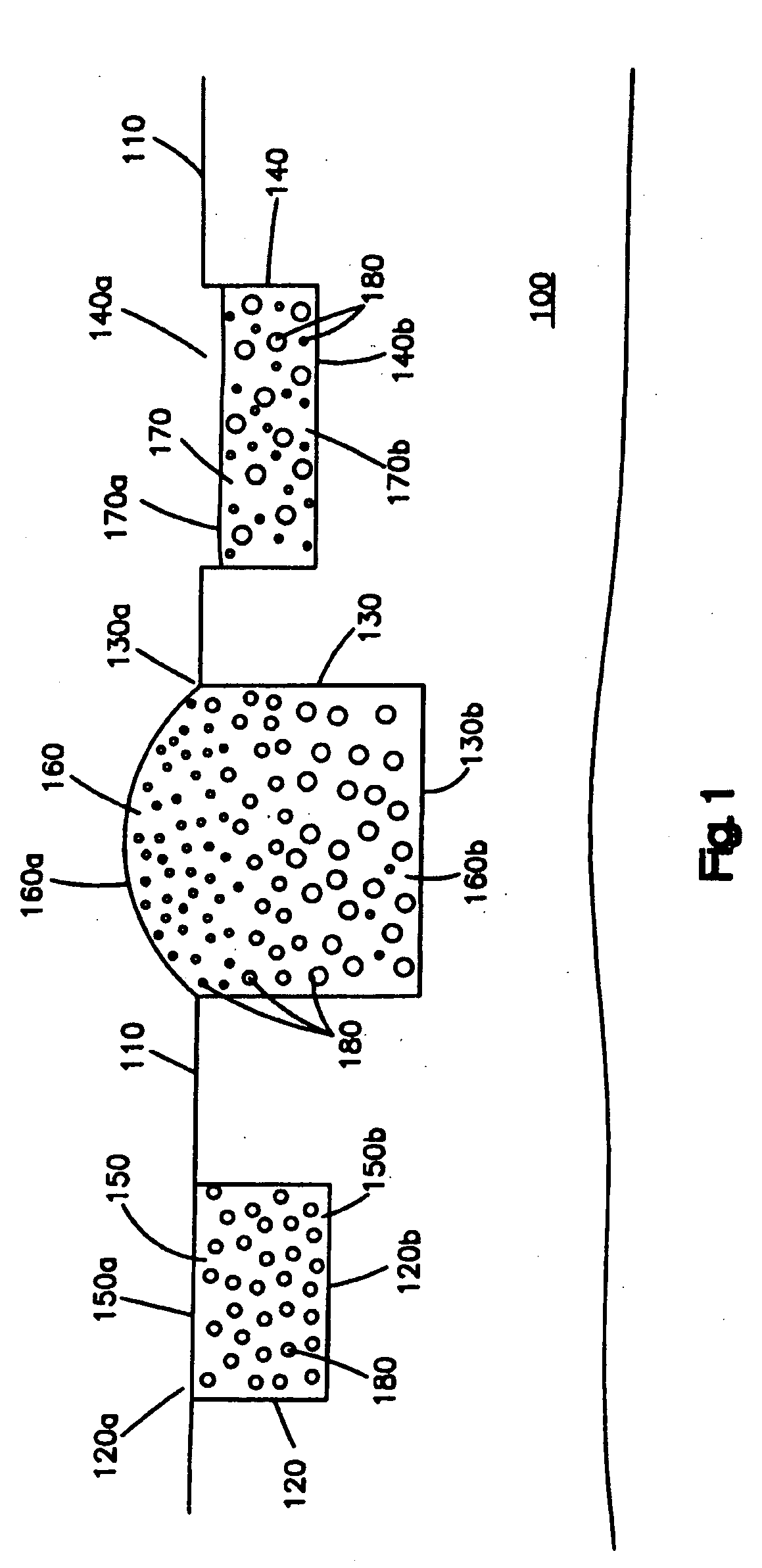
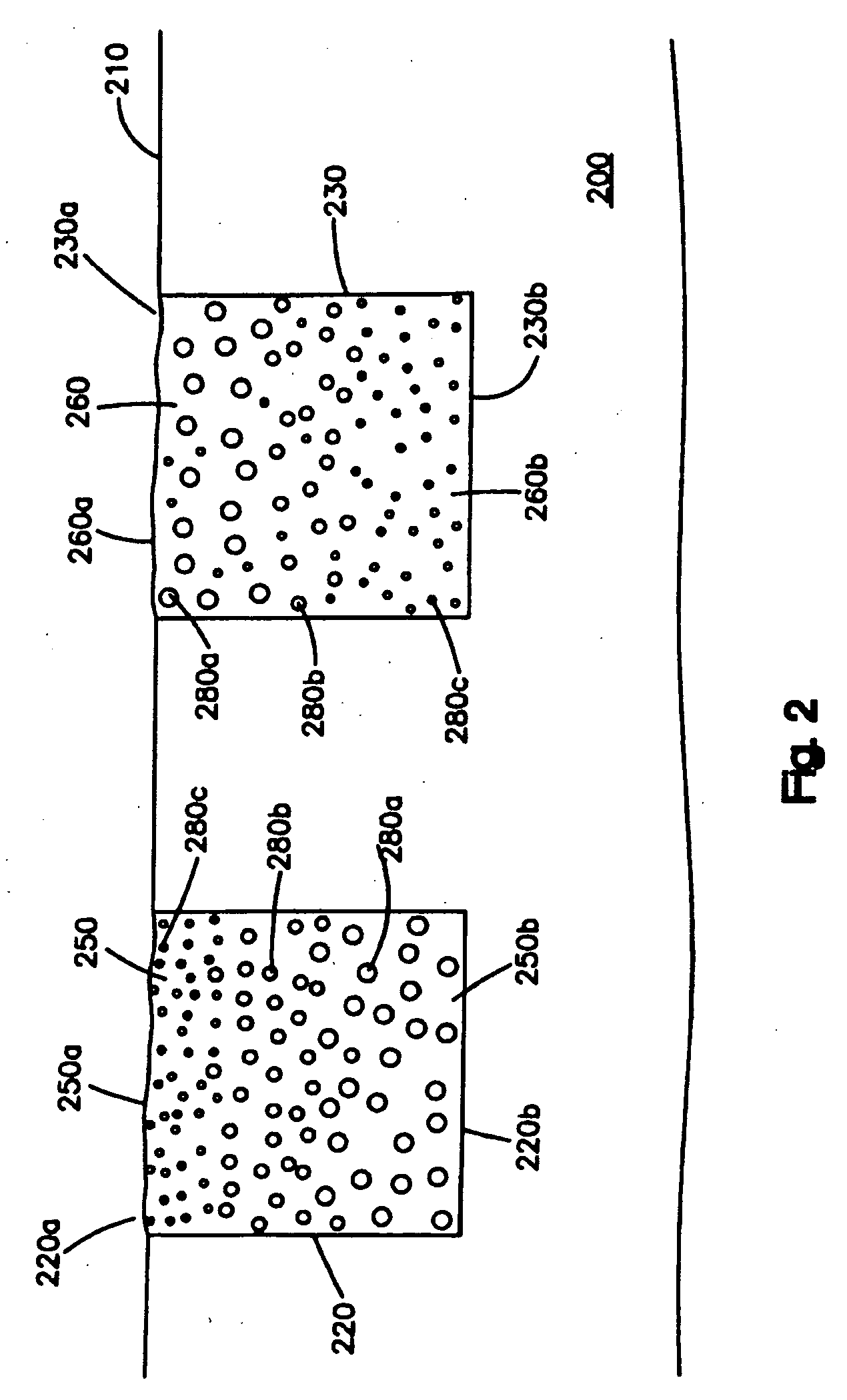
[0028]FIG. 1 shows one embodiment of the medical device, which can be a stent. The medical device comprises a substrate 100 having a surface 110. In this embodiment, the surface 110 is free of any coating, i.e., is not covered by a coating. In other embodiments, a coating may be disposed on at least a portion of the surface 110. As shown in the figure, there are three cavities 120, 130 and 140 disposed in the substrate 100. Each cavity comprises two op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com