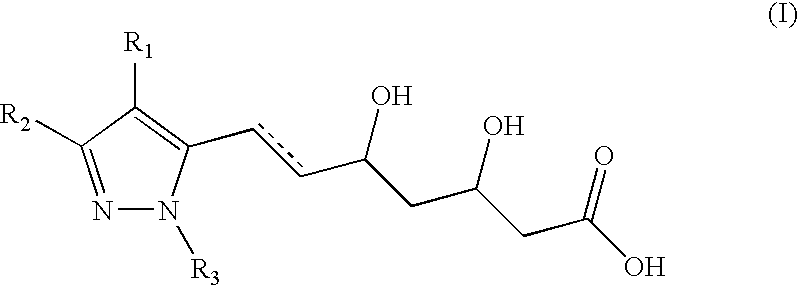
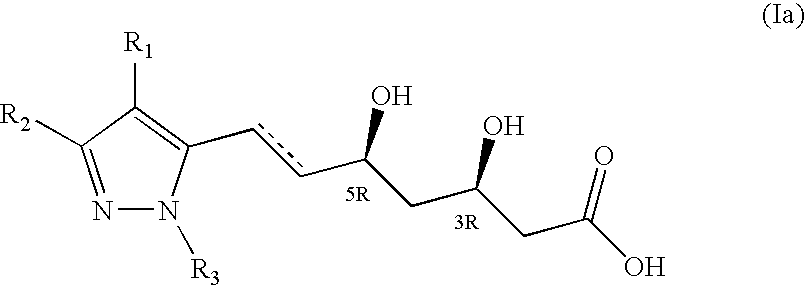
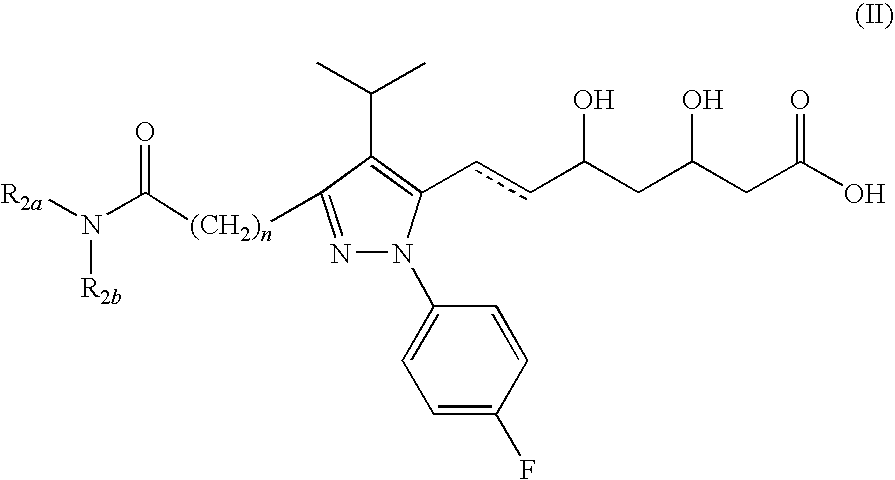
NOVEL PYRAZOLE-BASED HMG CoA REDUCTASE INHIBITORS
a technology of coa reductase inhibitor and pyrazole, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of statin interference with and/or inhibiting hmg-coa, early and rate-limiting step in conversion of hmg-coa to mevalonate,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(3R,5R)-7-[5-benzylcarbamoyl-2-(4-fluoro-phenyl)-4-isopropyl-2H-pyrazol-3-yl]-3,5-dihydroxy-heptanoic acid sodium salt
[0316]
(a) Step A. Preparation of [(4-Fluoro-phenyl)-hydrazono]-chloroacetic acid methyl ester
[0317]
(Reference: Tetrahedron Asymmetry 1999, 4447-4454): To a solution of 4-fluoroaniline (10.0 g, 90.0 mmol; commercially available from Sigma Aldrich) in MeOH (80 mL) was added 6 N HCl (80 mL) and the solution was cooled to 0° C. NaNO2 (12.4 g, 180 mmol) was then slowly added as a solid. The reaction was stirred for 15 min at 0° C. after which time NaOAc was added as a solid to adjust the reaction to pH 5. Subsequently, a solution of methyl 2-chloroacetoacetate (10.96 mL, 90.0 mmol; commercially available from Sigma Aldrich) in MeOH (40 mL) was slowly added at 0° C. The reaction was then allowed to warm to 25° C. and stirred for 12 hr after which time the MeOH was removed under reduced pressure and ether (300 mL) was added. The organic layer was separated and washed with s...
example 2
(3R,5R)-7-[2-(4-fluoro-phenyl)-4-isopropyl-5-(2-methyl-benzylcarbamoyl)-2H-pyrazol-3-yl]-3,5-dihydroxy-heptanoic acid sodium salt
[0343]
[0344]The title compound was prepared in a manner analogous to the method of Example 1. MS (APCI+): m / z 510.2 (M−H); H-NMR (DMSO-d6) δ 7.53-7.50 (m, 2H), 7.30 (t, 2H), 7.20-7.17 (m, 1H), 7.08-7.05 (m, 3H), 4.33 (s, 3H), 3.61-3.59 (m, 1H), 3.45-3.44 (m, 1H), 3.25 (bs, 1H), 2.73-2.67 (m, 1H), 2.58-2.51 (m, 1H), 2.24 (s, 3H), 1.94-1.89 (m, 1H), 1.75-1.69 (m, 1H), 1.37-1.10 (m, 10H).
example 3
(3R,5R)-7-[2-(4-fluoro-phenyl)-4-isopropyl-5-(3-methyl-benzylcarbamoyl)-2H-pyrazol-3-yl]-3,5-dihydroxy-heptanoic acid sodium salt
[0345]
[0346]The title compound was prepared in a manner analogous to the method of Example 1: MS (APCI+): m / z 512.2 (M+H); H-NMR (DMSO-d6) δ 7.53-7.49 (m, 2H), 7.30 (t, 2H), 7.14-6.95 (m, 4H), 4.30 (s, 2H), 3.62-3.55 (m, 1H), 3.46-3.42 (m, 1H), 3.26-3.19 (m, 1H), 2.69-2.61 (m, 1H), 2.58-2.51 (m, 1H), 2.21 (s, 3H), 1.91-1.88 (m, 1H), 1.72-1.66 (m, 1H), 1.34-1.10 (m, 10H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com