Neurotrophic components of the adnf i complex
a neurotrophic factor and complex technology, applied in the field of activity dependent neurotrophic factor complexes, can solve the problems of severe debilitating conditions, and achieve the effects of enhancing the activity of components i, blocking the survival-promoting activity, and enhancing the survival-promoting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0220]The following examples are offered to illustrate, but not to limit the claimed invention.
example i
Demonstration of Additional Subunits or Accessory Components of ADNF I Complex
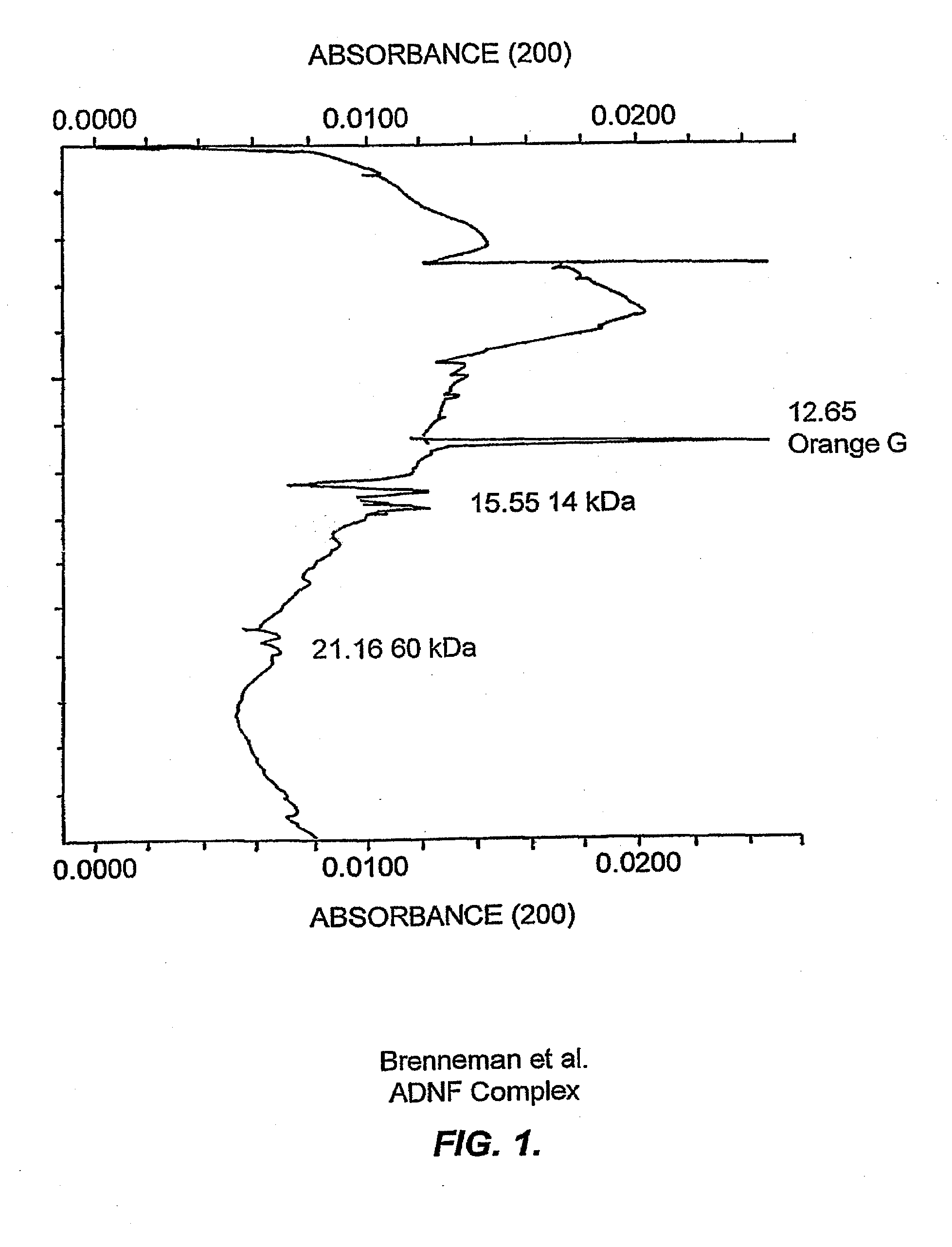
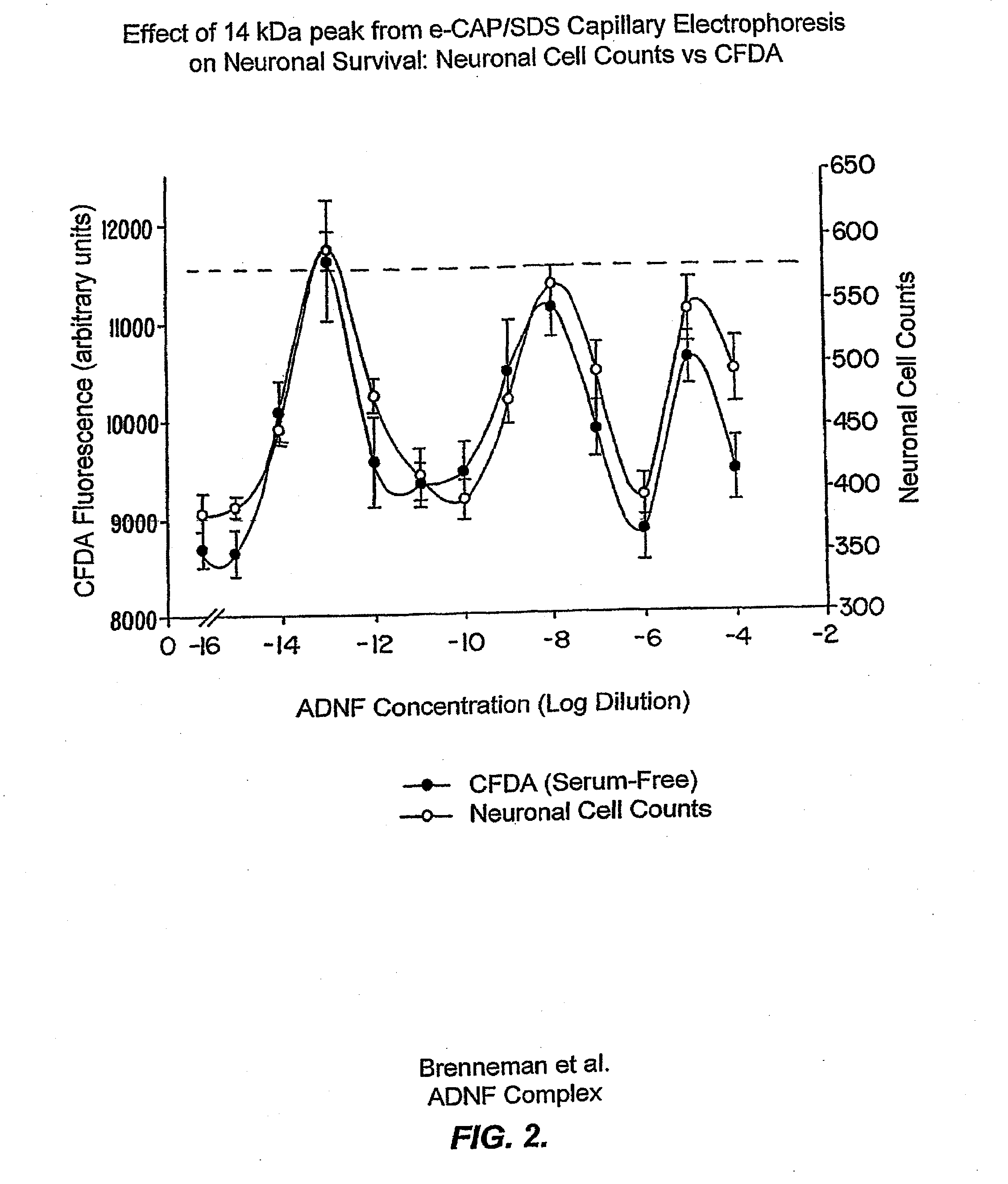
[0221]Evidence is presented that purified ADNF I, when analyzed under acidic conditions or when treated with a protease inhibitor cocktail (see Example III), exhibited a complex dose response for survival-promoting activity that indicates the existence of labile and novel components that were not previously identified. These data are consistent with the existence of labile components of the ADNF I complex that are only apparent when a serine protease inhibitor is present or when the pH is lowered to 4.5.
[0222]ADNF I was isolated as previously described (see, e.g., Brenneman & Gozes, J. Clin. Invest. 97:2299-2307 (1996)) and then desalted and stored in 10 mM phosphate buffer (pH 4.5). The purified ADNF I was diluted 1:3 with water and analyzed on eCAP / SDS capillary electrophoresis (CE). The running and sample buffers for these analyses were components of the eCAP / SDS kit (Beckmann Instruments). Electrophore...
example ii
Demonstration of Tryptic Digest Peptides from ADNF I Complex and their Neuroprotective Activity in CNS Cultures
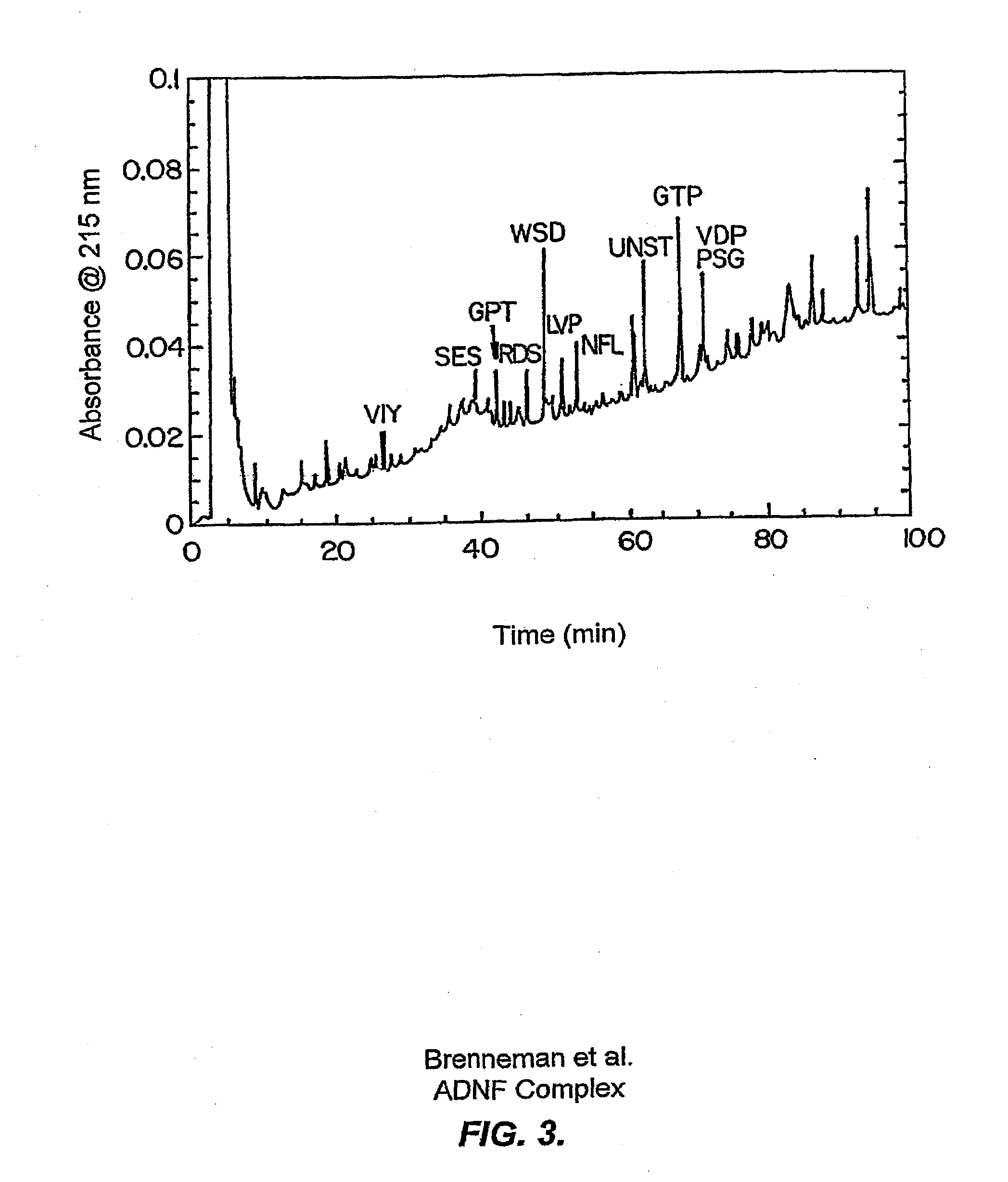
[0224]Digest peptides were obtained after treatment of purified ADNF I with trypsin as previously described (Williams & Stone, Techniques in Protein Chemistry VI. pp 79-90 (1997)). As shown in FIG. 3, the peptides were separated by reverse phase HPLC. The peptide identified for each of the peaks is given as the first three amino acids of the peptide. Aliquots of the isolated peptides were sequenced by Edman degradation or tested for survival-promoting activity in TTX-treated cerebral cortical cultures. As shown in FIG. 4, three digest peptide fractions exhibited survival-promoting activity in vitro. The active peptides were:
[0225]1. NNSTTYAPISANVSTALGSTAALPTAAGPV (SEQ ID NO:2) (retention time: 62 min) Abrev.: NNST
[0226]2. GPTADITLTK (SEQ ID NO:7) (retention time: 43 min) Abrev.: GPT
[0227]3. NPSGTDWLNTNNQANPFN (SEQ ID NO:4) (retention time: 71 min) Abrev. PSG
[0228]One additi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| constant voltage | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com