Novel Combinations of DNAK Inhibitors With Known Antibacterial Agents
a technology of dnak inhibitors and antibacterial agents, which is applied in the field of natural peptide dnak inhibitors, can solve the problems of increased tendency to induce bacterial resistance, poor toxicological profiles, and poor physiochemical properties, and achieves the effects of extending the duration of activity, lowering the necessary therapeutically effective dose, and improving antibacterial potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
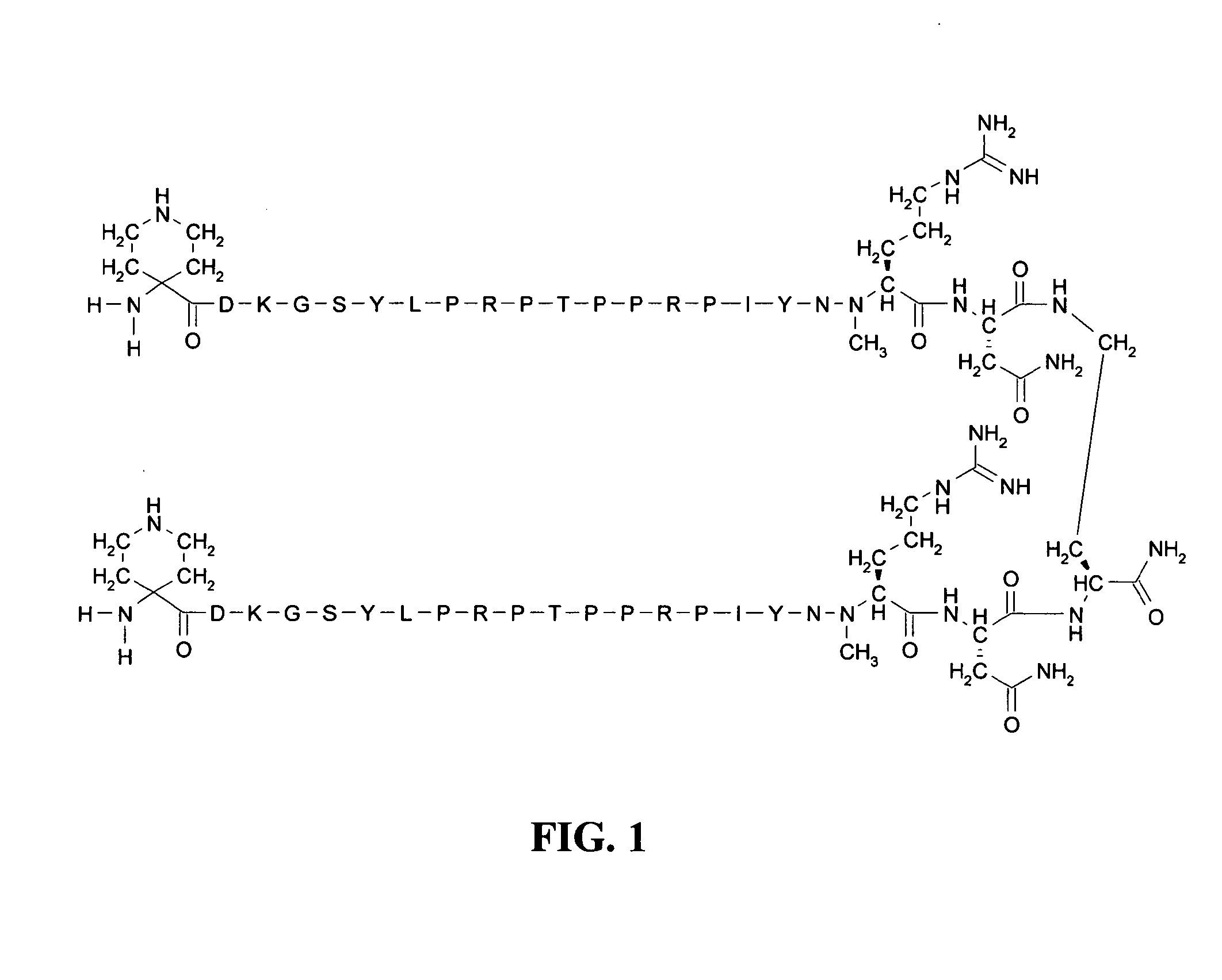
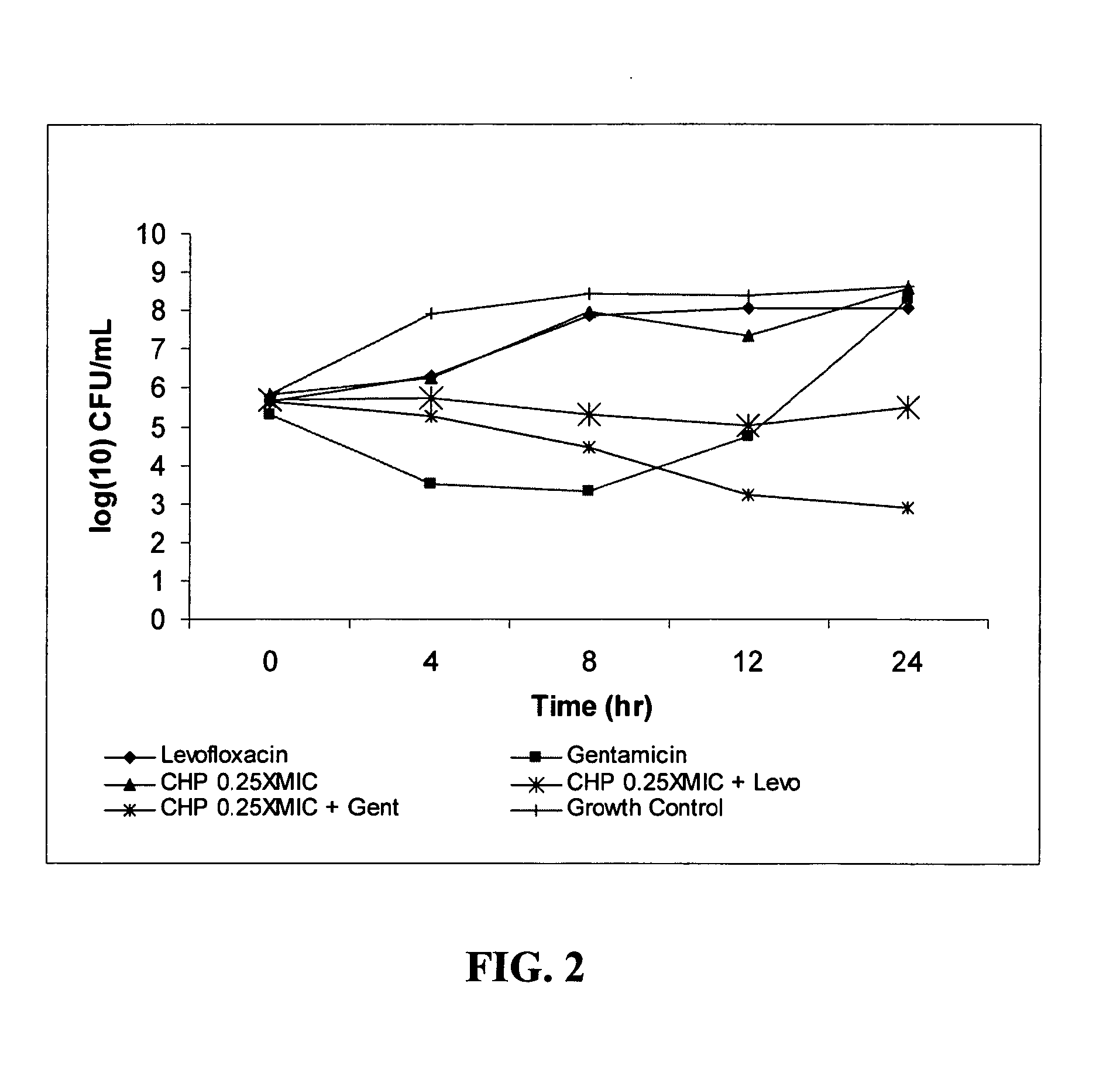
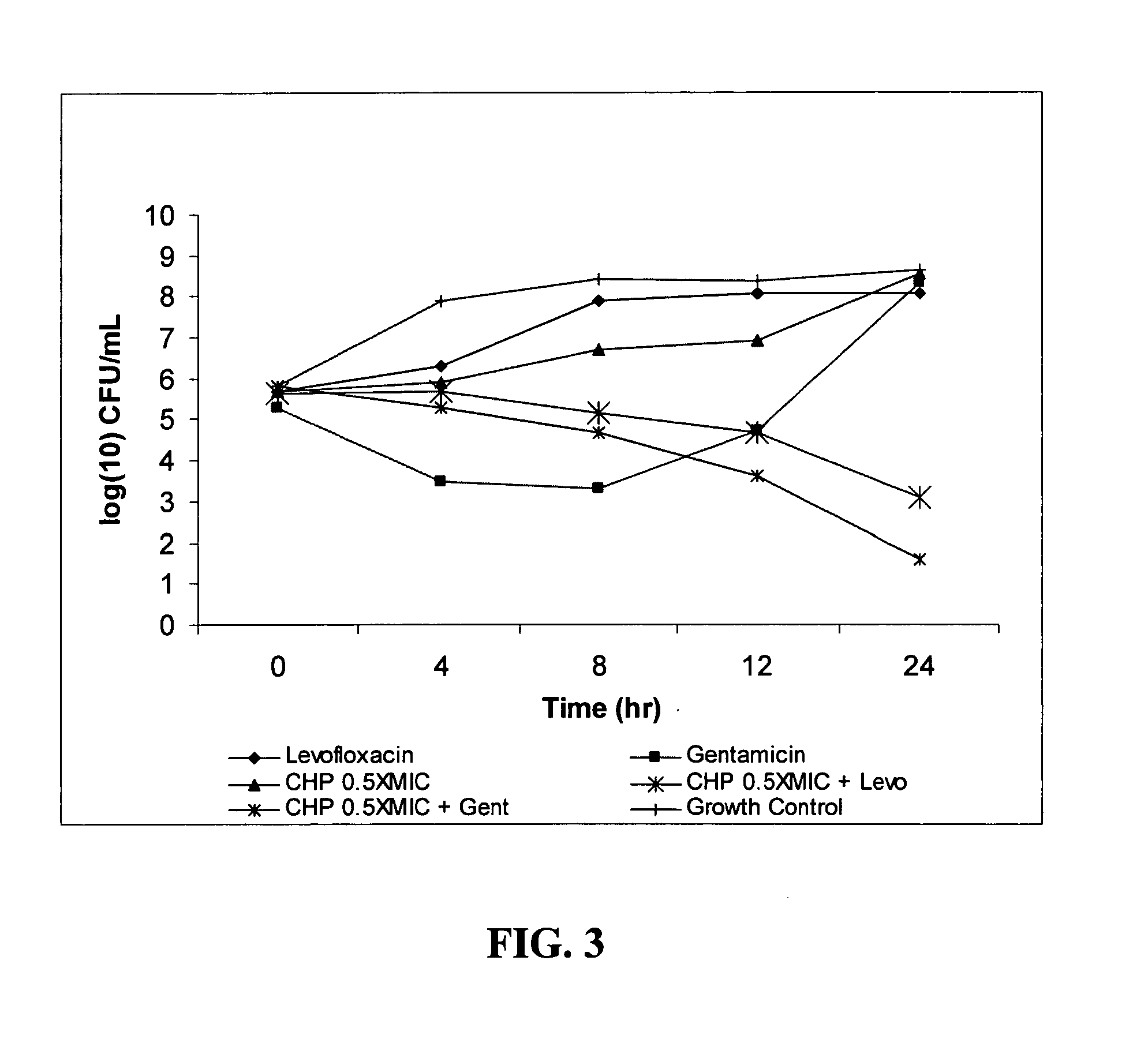
[0099]Growth inhibition assays were performed using the Gram negative E. coli isolate CHP-04-002EC obtained from Laboratory Specialists, Inc. (Westlake, Ohio). DnaK inhibitor CHP-105 (structure shown in FIG. 1) at concentrations of 16 μg / ml (0.25×MIC; FIG. 2), 32 μg / ml (0.5×MIC; FIG. 3), 64 μg / ml (1×MIC; FIG. 4), 128 μg / ml (2×MIC; FIG. 5) and 256 μg / ml (4×MIC; FIG. 6) were applied to midlogarithmic phase bacterial culture, either alone or in combination with levofloxacin (0.125 μg / ml; 0.25×MIC) or gentamicin (0.06 μg / ml; 0.25×MIC). Untreated bacterial culture served as growth control. At 4, 8, 12 and 24 hrs post-application, the cultures were serially diluted, plated and allowed to grow overnight. Colony forming units (CFU) were determined by counting the number of newly grown colonies and multiplying by the dilution factor. A >1 log decrease in CFU compared to the most active single agent is considered additive, while a >2 log decrease in CFU is considered synergistic.
[0100]As can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com