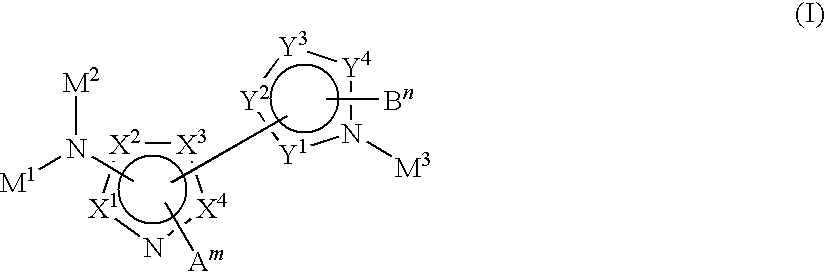
Novel heteroaromatic derivatives and their use as positive allosteric modulators of metabotropic glutamate receptors
a technology of metabotropic glutamate receptor and allosteric modulation, which is applied in the direction of drug composition, biocide, metabolic disorder, etc., can solve the problem of glutamatergic neurotransmission imbalan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(2-Chlorophenyl)-3-(3-methyl-1H-pyrazol-4-yl)-1,2,4-thiadiazol-5-amine (Final Compound 1-3)
tert-Butyl 4-cyano-3-methyl-1H-pyrazole-1-carboxylate
[0156]According to Scheme 1 Step 1: To a solution of 3-methyl-1H-pyrazol-4-carbonitrile (9.34 mmol, 1.00 g) in DCM (20 mL) were sequentially added DIEA (9.34 mmol, 1.60 mL), (Boc)2O (9.34 mmol, 2.04 g) and DMAP (0.93 mmol, 0.11 g). After stirring for 14 hours at room temperature, the reaction mixture was quenched with water. The aqueous phase was extracted with DCM. The organic phase was washed with a saturated solution of NaHCO3 and brine, was dried over MgSO4, was filtered and was concentrated under reduced pressure. The crude product was purified by flash chromatography over silica gel using cyclohexane / AcOEt (85:15) as eluent to afford tert-butyl 4-cyano-3-methyl-1H-pyrazole-1-carboxylate (7.96 mmol, 1.65 g, 85%) as a white solid.
[0157]LC (Zorbax SB-C18, 3.5 μm, 4.6×50 mm Column): RT=3.72 min; MS m / z ES+=108 (M+-Boc).
3-Methyl-1H-pyrazo...
example 2
3-(3-Propyl-1H-pyrazol-4-yl)-N-(pyridin-2-yl)-1,2,4-thiadiazol-5-amine (Final Compound 1-9)
Ethyl 1-(4-methoxybenzyl)-3-propyl-1H-pyrazole-4-carboxylate and ethyl 1-(4-methoxybenzyl)-5-propyl-1H-pyrazole-4-carboxylate
[0166]According to Scheme 1 Step 1: A suspension of ethyl 5-propyl-1H-pyrazole-4-carboxylate (2.74 mmol, 500 mg), 1-(bromomethyl)-4-methoxybenzene (2.74 mmol, 0.39 mL) and K2CO3 (2.74 mmol, 379 mg) in acetonitrile (10 mL) was heated at 70° C. overnight. Water was added and the aqueous phase was extracted with AcOEt. The organic phase was washed with water and brine, was dried over Na2SO4, was filtered and was concentrated under reduced pressure. The crude product was purified by flash chromatography over silica gel using cyclohexane / AcOEt (80:20) as eluent to afford a mixture of ethyl 1-(4-methoxybenzyl)-3-propyl-1H-pyrazole-4-carboxylate and of ethyl 1-(4-methoxybenzyl)-5-propyl-1H-pyrazole-4-carboxylate (2.15 mmol, 650 mg, 78%) as an orange oil.
[0167]LC (Zorbax SB-C18,...
example 3
3-(3-Methyl-1H-pyrazol-4-yl)-N-(pyridin-2-yl)-1,2,4-thiadiazol-5-amine (Final Compound 1-5)
1-(4-Methoxybenzyl)-3-methyl-1H-pyrazol-4-carbonitrile and 1-(4-methoxybenzyl)-5-methyl-1H-pyrazol-4-carbonitrile
[0176]According to Scheme 1 Step 1: Triphenylphosphine (11 mmol, 2.9 g), (4-methoxyphenyl)methanol (10 mmol, 1.4 g) and di-tert-butylazodicarboxylate (11 mmol, 2.6 g) were added to a solution of 3-methyl-1H-pyrazol-4-carbonitrile (9.3 mmol, 1.0 g), in DCM (40 mL) at 0° C. The reaction mixture was stirred at room temperature overnight. The organic phase was washed with a saturated solution of NH4OH and brine. Then the organic phase was dried over MgSO4, was filtered and was concentrated under reduced pressure. The resulting crude product was purified by flash chromatography over silica gel using cyclohexane / AcOEt (90:10) as eluent to yield a mixture of 1-(4-methoxybenzyl)-3-methyl-1H-pyrazol-4-carbonitrile and of 1-(4-methoxybenzyl)-5-methyl-1H-pyrazol-4-carbonitrile (9.3 mmol, 2.1 g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com