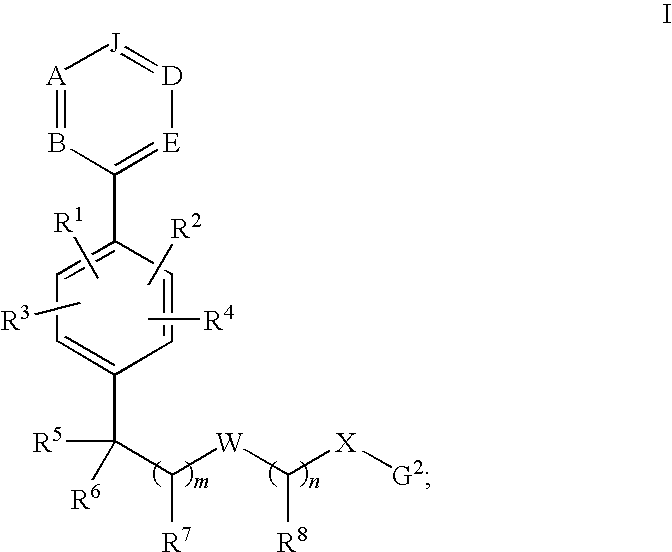
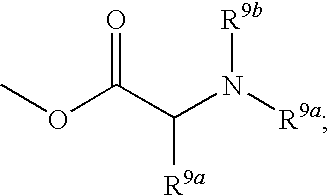
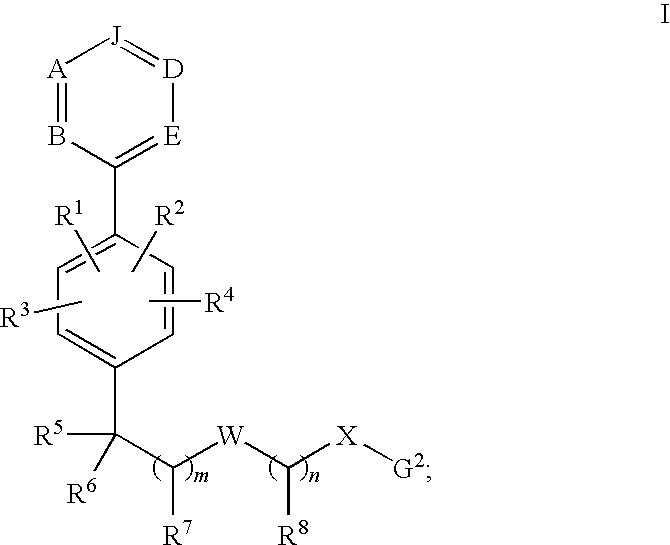
Phenyl derivatives and methods of use
a technology of phenyl derivatives and derivatives, applied in the field of new phenyl compounds, can solve the problems of causing a number of unwanted cns side effects, separating clinically undesirable psychotropic effects from therapeutically desirable effects on the peripheral nervous system, immune and endocrine system,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-(3-(Benzyloxy)phenyl)-2-methylpropanenitrile (II)
[0338]
[0339]A solution of commercially available nitrile I (83.9 g, 0.38 mol) in DMSO (57 mL) and 50% NaOH solution (120 mL) were simultaneously added to DMSO (425 mL) previously saturated for one hour with bromomethane gas. Bromomethane was continuously bubbled through the reaction mixture during the addition and then for a further 1.5 hours with ice-cooling to maintain the temperature at 50° C. or less. The reaction mixture was added to a 600 mL H20-600 g ice mixture and then extracted with Et2O (3×800 mL), the ethereal layers were washed with water (1L) and brine (1L), dried and concentrated under reduced pressure to give a yellow oil. The yellow oil was cooled in a dry-ice acetone bath until it solidified and then left to stand at room temperature overnight to afford II as a light yellow crystalline solid (93.59 g, 98%). M+1=252.
example 2
Preparation of 2-(3-(benzyloxy)phenyl)-2-methylpropanal (III)
[0340]
[0341]Diisobutylaluminum hydride (300 mL, 0.3 mol of a 1.0 M solution in hexanes) was added dropwise to a cooled (15° C., ice-bath) solution of nitrile II (59.9 g, 0.24 mol) in anhydrous tetrahydrofuran (250 mL). The reaction temperature was maintained at 15-18° C. during the addition. The reaction was then allowed to warm to room temperature and stirred for an additional 2 hours. The reaction was hydrolyzed by addition of a cold solution of conc. H2SO4 (35.5 mL) in water (117.5 mL) with the temperature maintained at <30° C. The resultant mixture was stirred for a further 2 hours, then filtered, and the filtrate extracted with diethyl ether (2×250 mL), washed with water (300 mL), brine (300 mL), dried and concentrated under reduced pressure to give crude aldehyde III as a light-yellow oil (59.82 g, 98%) which was used without further purification. M+1=255.
example 3
Preparation of 2-(3-Benzyloxy-phenyl)-2-methyl-propan-1-ol (IV)
[0342]
[0343]Reduction of III was carried out under standard conditions using NaBH4 in tetrahydrofuran as described by Chaikin and Brown (J. Am. Chem. Soc. 1949, 71, 122) to generate IV quantitatively. M+1=257
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com