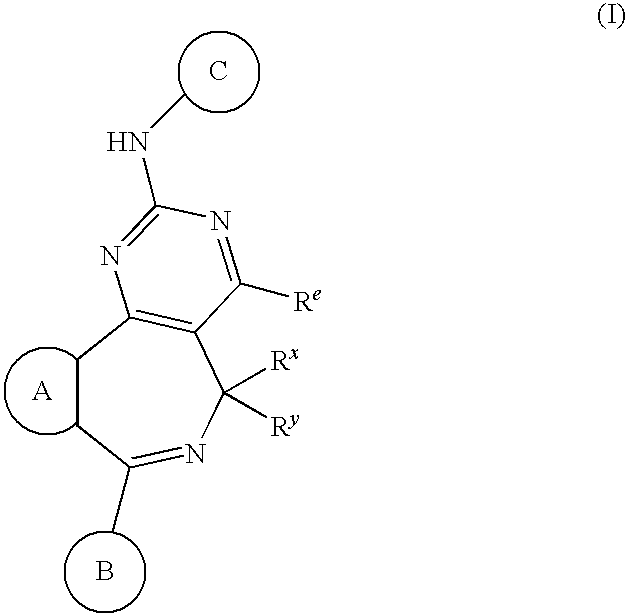
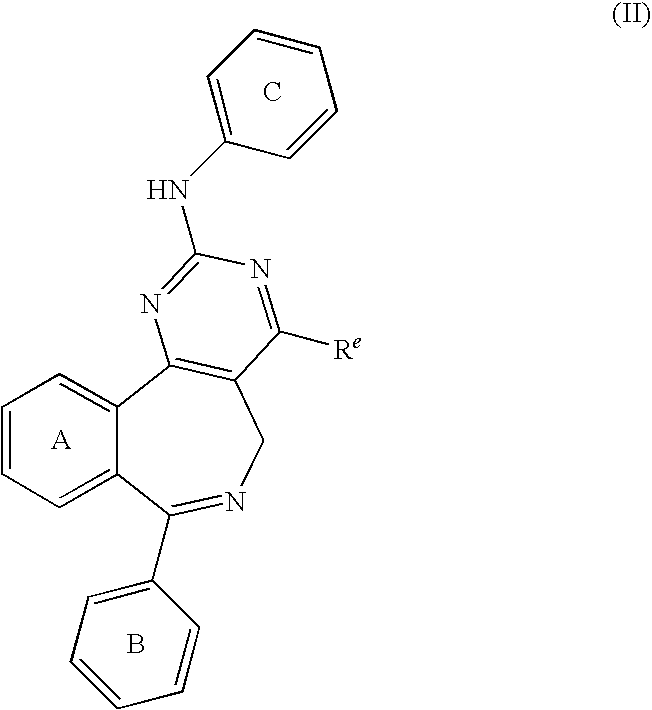
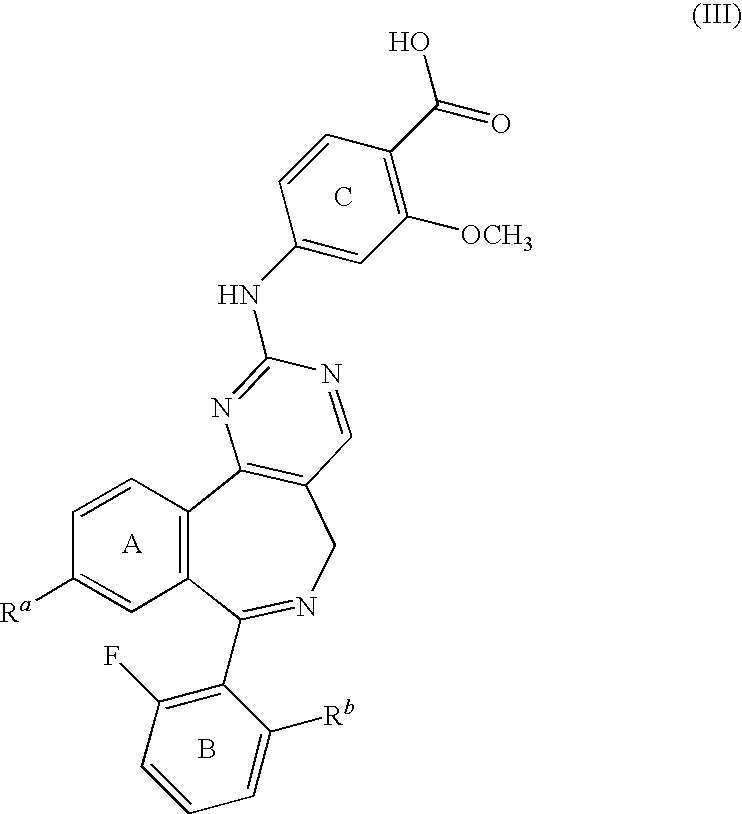
Combination of Aurora Kinase Inhibitors and Anti-CD20 Antibodies
a technology of aurora kinase inhibitors and anti-cd20 antibodies, which is applied in the field of methods for the treatment of hematological malignancies, can solve the problems of often subjected patients to disease relaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Combination of Aurora A Kinase Specific Inhibitor (Compound III-1) and Rituximab in a Sub-Cutaneous Ly19 Lymphoma Model Grown in Female SCID Mice
Experimental Overview
[0164]This is an in vivo experiment looking at tumor volume after treatment with the combination of Compound III-1 and rituximab. Tumor growth was monitored with vernier calipers. The mean tumor volume was calculated using the formula V=W2×L / 2. When the mean tumor volume reached approximately 200 mm3, the animals were randomized into the following eight treatment groups, with each cohort made up of ten mice:
[0165]Vehicle
[0166]3 mg / kg Compound III-1 (qd)
[0167]10 mg / kg Compound III-1 (qd)
[0168]10 mg / kg Compound III-1 (bid)
[0169]10 mg / kg rituximab (qw)
[0170]3 mg / kg Compound III-1 (qd)+10 mg / kg rituximab (qw)
[0171]10 mg / kg Compound III-1 (qd)+10 mg / kg rituximab (qw)
[0172]10 mg / kg Compound III-1 (bid)+10 mg / kg rituximab (qw)
[0173]The animals were inoculated with 4.0×106 cells from cell line Ly-19 at implant site Flank (cell ...
example 2
Combination of Aurora A Kinase Specific Inhibitor (Compound III-1) and Rituximab in a Subcutaneous WSU-Luc Lymphoma Model Grown in Female SCID Mice
Experimental Overview
[0186]This is an in vivo experiment looking at tumor volume after treatment with the combination of Compound III-1 and rituximab. Tumor growth was monitored with vernier calipers. The mean tumor volume was calculated using the formula V=W2×L / 2. When the mean tumor volume reached approximately 250 mm3, the animals were randomized into the following six treatment groups, with each group made up of ten mice:
[0187]Vehicle
[0188]3 mg / kg Compound III-1 (P.O., qd)
[0189]10 mg / kg Compound III-1 (P.O., qd)
[0190]10 mg / kg rituximab (I.V., q7d)
[0191]3 mg / kg Compound III-4+10 mg / kg rituximab
[0192]10 mg / kg Compound III-1+10 mg / kg rituximab
[0193]The animals were inoculated with 4.0×106 cells from cell line WSU-DLCL2 at implant site Flank (cell suspension). Compounds were administered for 21 days, and tumor volumes were measured on day...
example 3
Combination of Aurora A Kinase Specific Inhibitor (Compound III-1) and Rituximab in a Sub-Cutaneous Primary Diffuse Large B-Cell Lymphoma Model (PHTX-22-06) Grown in Female SCID Mice
Experimental Overview
[0199]This is an in vivo experiment looking at tumor volume after treatment with the combination of Compound III-1, and rituximab. Tumor growth was monitored with vernier calipers. The mean tumor volume was calculated using the formula V=W2×L / 2. When the mean tumor volume reached approximately 200 mm3, the animals were randomized into the following six treatment groups, with each group made up of ten mice:
[0200]Vehicle
[0201]10 mg / kg Compound III-4 (P.O., bid)
[0202]20 mg / kg Compound III-1 (P.O., bid)
[0203]10 mg / kg rituximab (I.V., q7d)
[0204]10 mg / kg Compound III-1+10 mg / kg rituximab
[0205]20 mg / kg Compound III-1+10 mg / kg rituximab
[0206]The animals were inoculated with 2×5 mm3 tumor mass from primary PHTX-22L-6 tumor chunk at implant site Flank (trocar). Compounds were administered for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com