Therapeutic formulations containing venom or venom Anti-serum either alone or in combination for the therapeutic prophylaxis and therapy of neoplasms
a technology of venom and anti-serum, which is applied in the direction of snake antigen ingredients, enzymes, biochemical equipment and processes, etc., can solve the problems of irreversible cell injury and eventually cell death, and achieve enhanced anti-cancer effects and prevent the growth and spread of neoplastic tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
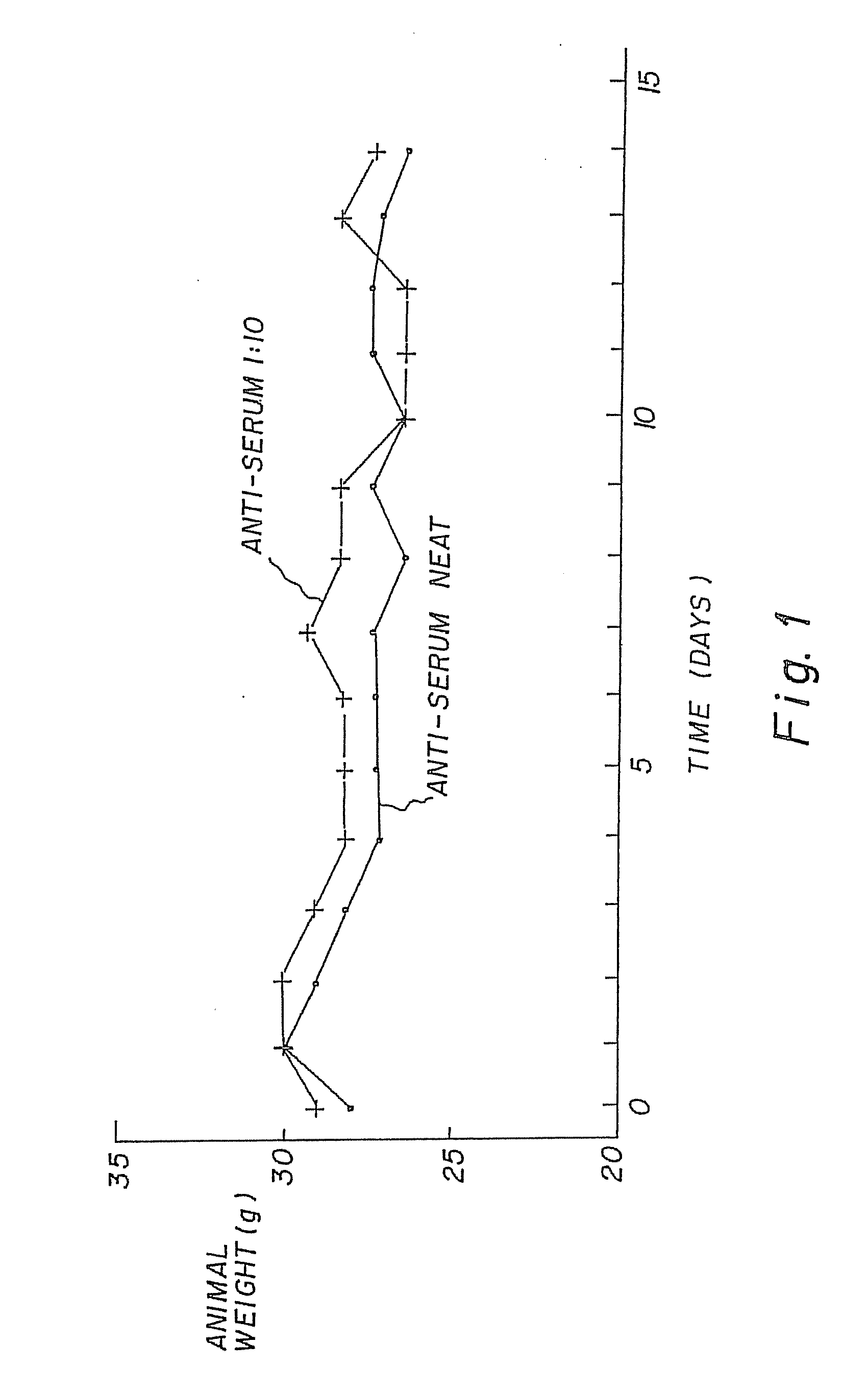
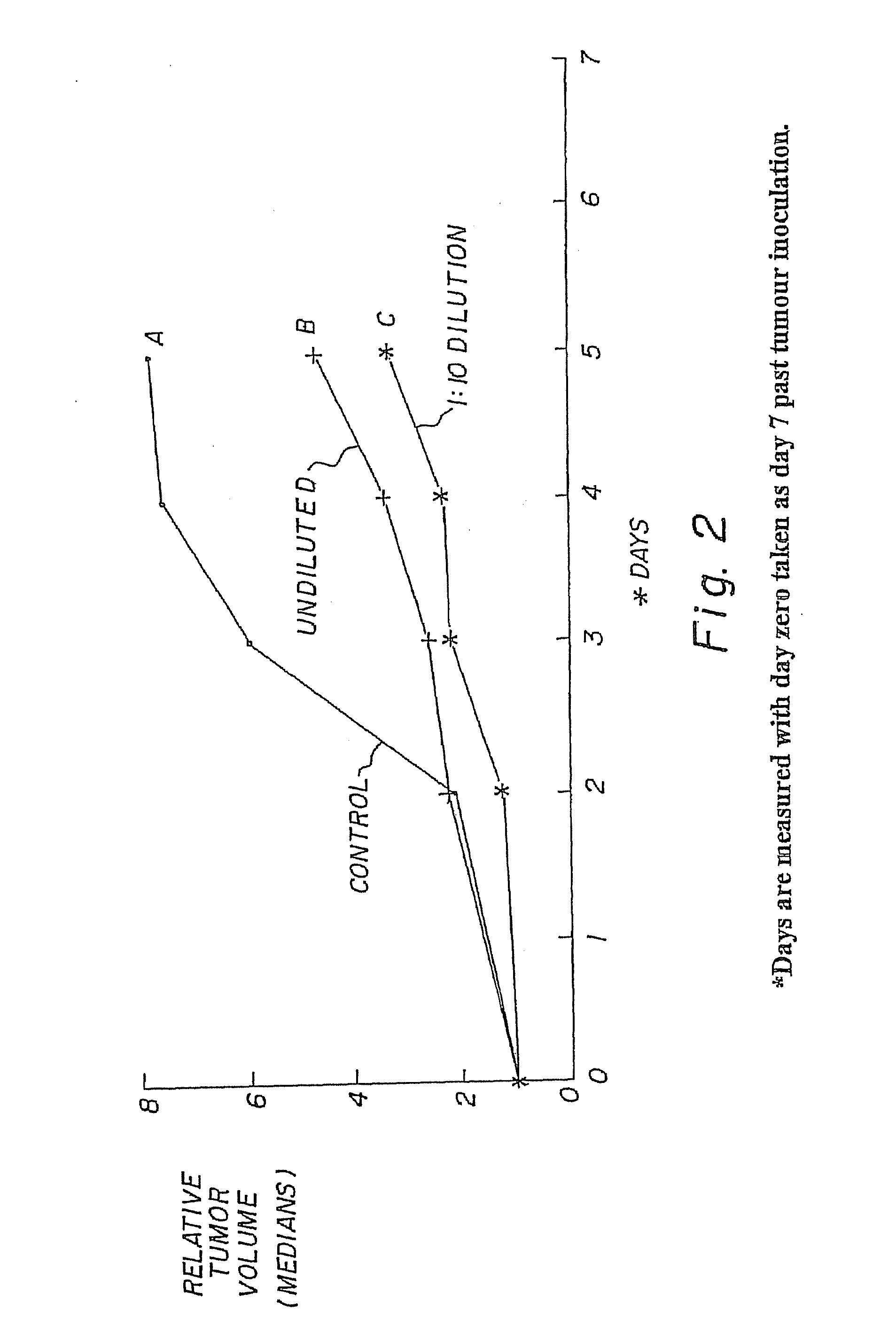
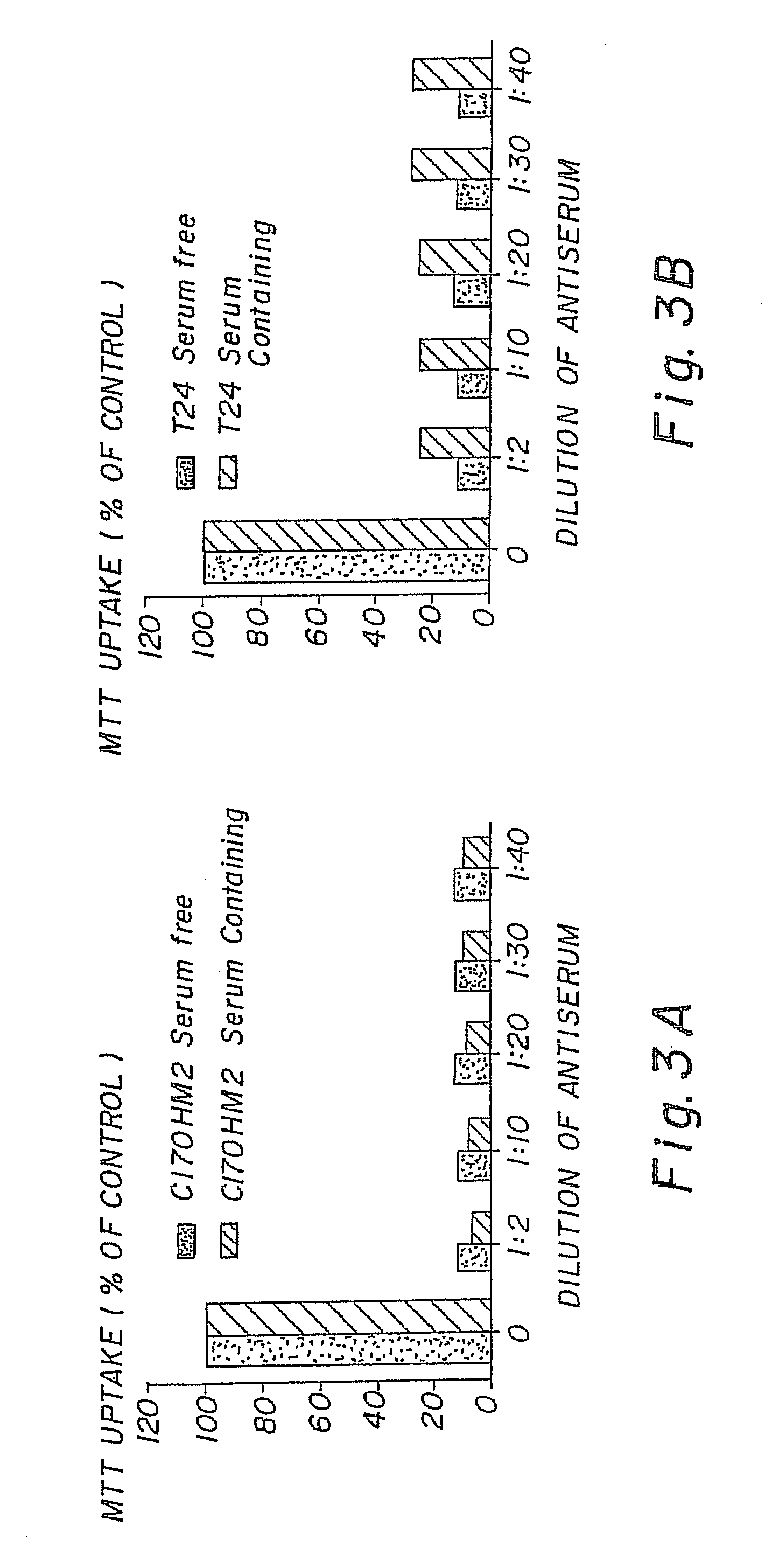
[0044]The therapeutic activity of the compounds of this invention are demonstrated by inhibition of the tumour cell lines in-vitro and in-vivo. The compounds were tested for toxicity in Scid mice. Results as in FIG. 1 [toxicity data].
Toxicity Study
Method
[0045]Female Scid mice (6-8 weeks of age) were treated with either a Neat or a 1:10 dilution of the anti-serum preparation, subcutaneously (0.1 ml, daily) for a period of 14 days. The weights of the mice were measured daily. At termination, organs were removed and fixed in formalin for histological examination.
Results
[0046]No toxicity, as assessed by animal weights and clinical well-being, was evident (FIG. 1).
[0047]The compounds of this invention may be combined with other known anti-inflammatory / immunosuppressive or chemotherapeutic agents such as steroids or non-steroidal anti-inflammatory agents in the pharmaceutical compositions and methods described herein.
[0048]Anti-serum to snake and / or insect venoms and / or mammalian and / or P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com