Methods of determining responsiveness to Anti-tnf alpha therapy in inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generally
[0069]Genetics, immune responses and environmental factors for disease susceptibility and development, as well as their interactions, are important determinants of inflammatory bowel disease phenotype and disease progression. These factors may also interact in such a way that influences the outcome of therapies used to treat these heterogeneous phenotypes. Recent genomic discoveries from Genome Wide Association (GWA) studies in both Crohn's disease (CD) and ulcerative colitis (UC) have increased understanding of the genetic susceptibility to IBD. This novel genetic information provides important insight regarding the various mechanisms of inflammation involved in disease pathogenesis. Targeting these various pathways with effective therapies is the key to the successful management of the IBD patient. When introduced, the monoclonal antibodies targeting tumor necrosis factor alpha (TNFα) represented the largest advance in decades made in the realm of IBD therapeutics. Howeve...
example 2
Significance of Defining Predictors of Response to Anti-TNFα
[0070]Defining predictors of response to anti-TNFα will allow clinicians to choose the appropriate therapy for the appropriate IBD patient with the goal of maximizing efficacy and minimizing toxicity. Research described herein will allow the individualization of therapy based on who will or perhaps more importantly will not respond to different classes of therapeutic interventions currently available to IBD patients. The development of lymphoma, particularly a rare almost uniformly fatal sub-type of hepatosplenic T cell lymphoma in individuals receiving infliximab along with immunomodulators have resulted in clinicians wanting to carefully select those patients who are appropriate candidates for these therapies. The novel pharmacogenetic information described herein can not only improve the management of patients in the clinic with an existing anti-TNFα agent but also ultimately change the way large scale clinical trials ar...
example 3
Pharmacogenetic GWAS and Primary Non-Response
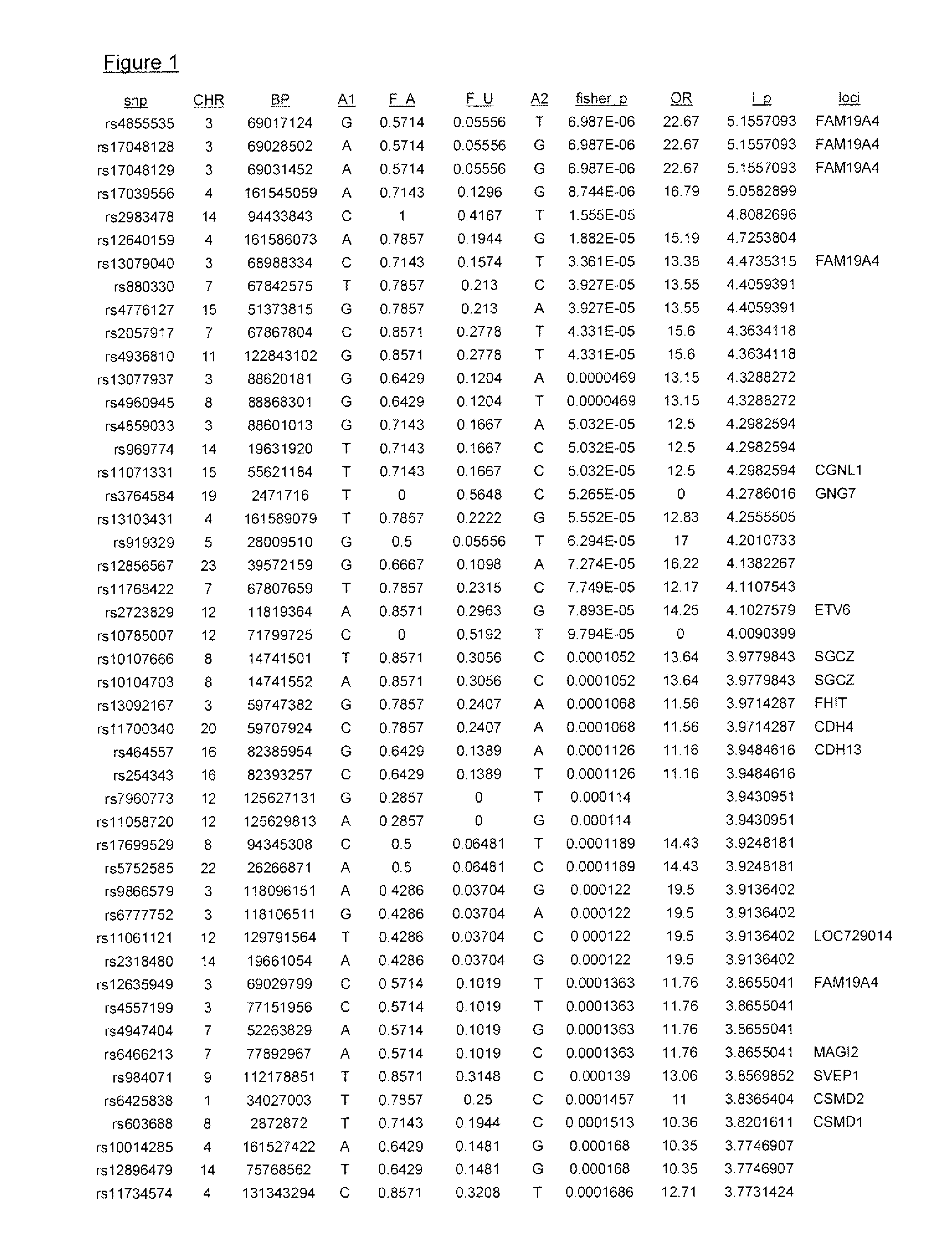
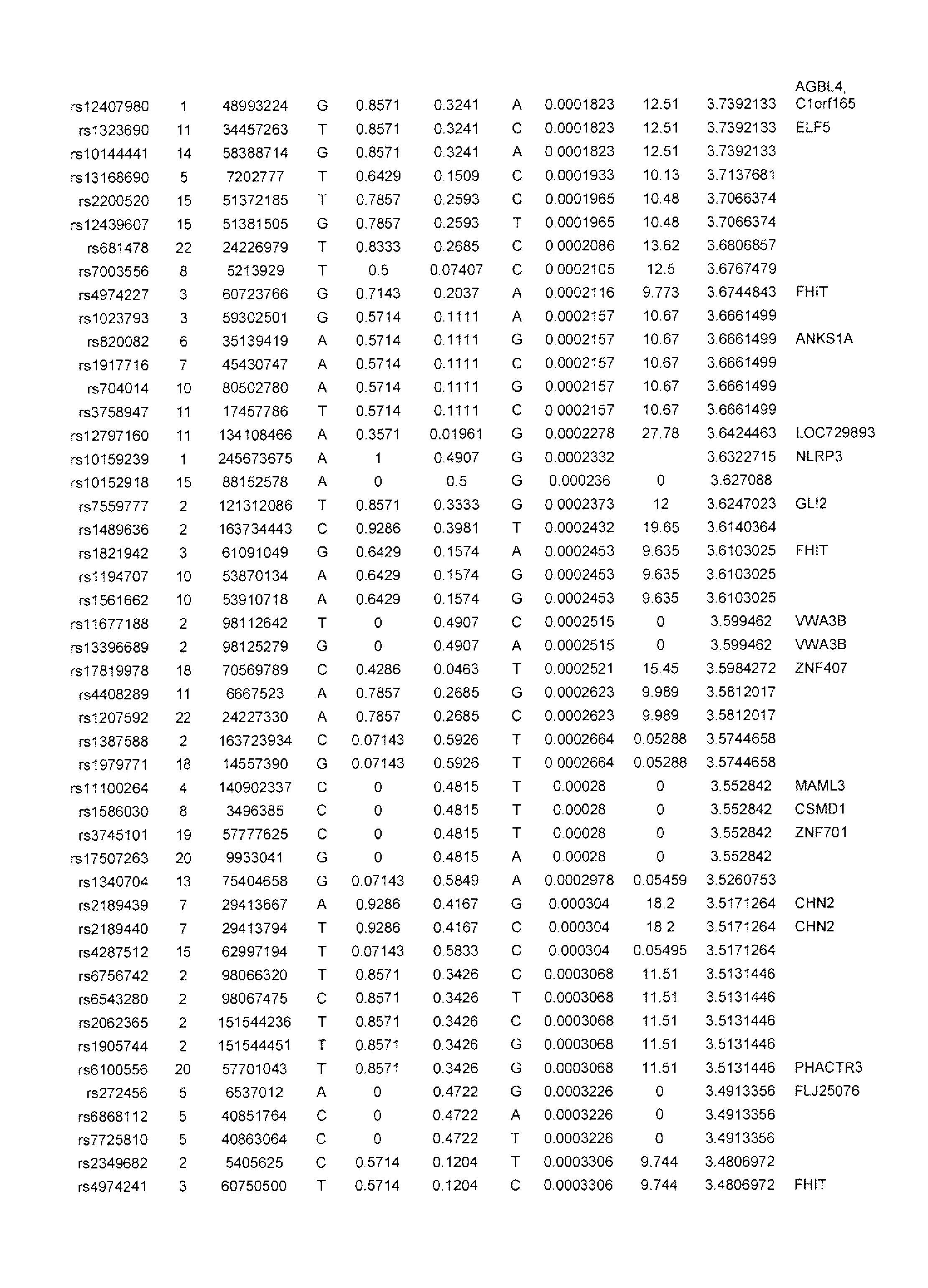
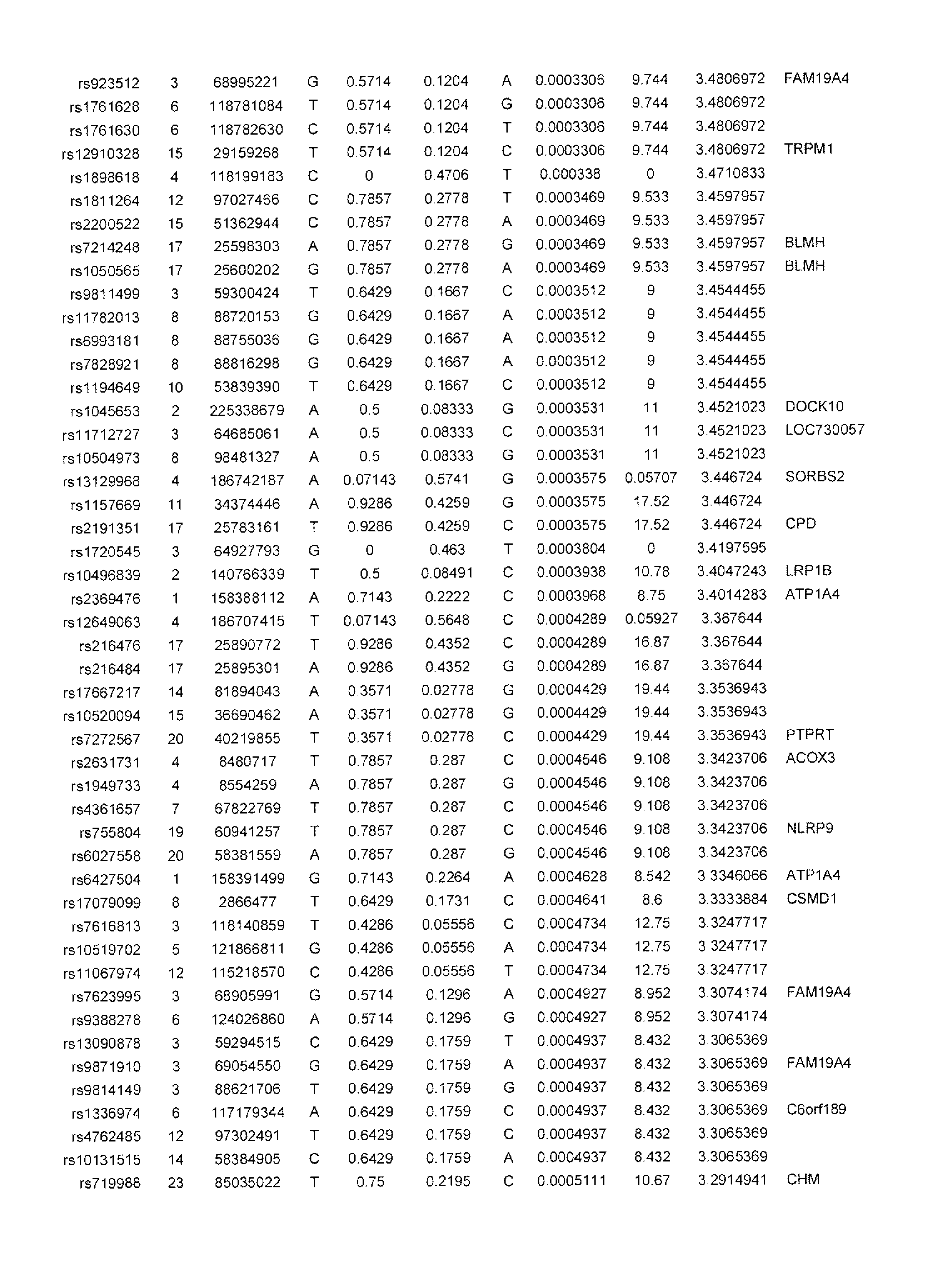
[0071]The inventors tested the association of the most significant CD susceptibility loci previously identified with infliximab responsiveness in pediatric IBD patients receiving infliximab from which there was complete clinical follow up. For these preliminary analyses, two (2) outcomes were evaluated:
[0072]1) primary non-response: patient did not respond to the induction regimen as defined by patient did not receive a clinical benefit from the first 3 infusions of infliximab and did not receive any further treatment doses. All significant associations are shown in Table 1 below. Remainder of analyses are detailed in Table 3.
[0073]2) secondary loss of response: patient responded to the induction regimen and despite dose escalation and / or frequency intensification of infliximab the drug was discontinued as of last follow up. Time to loss of response was also analyzed and data are shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com