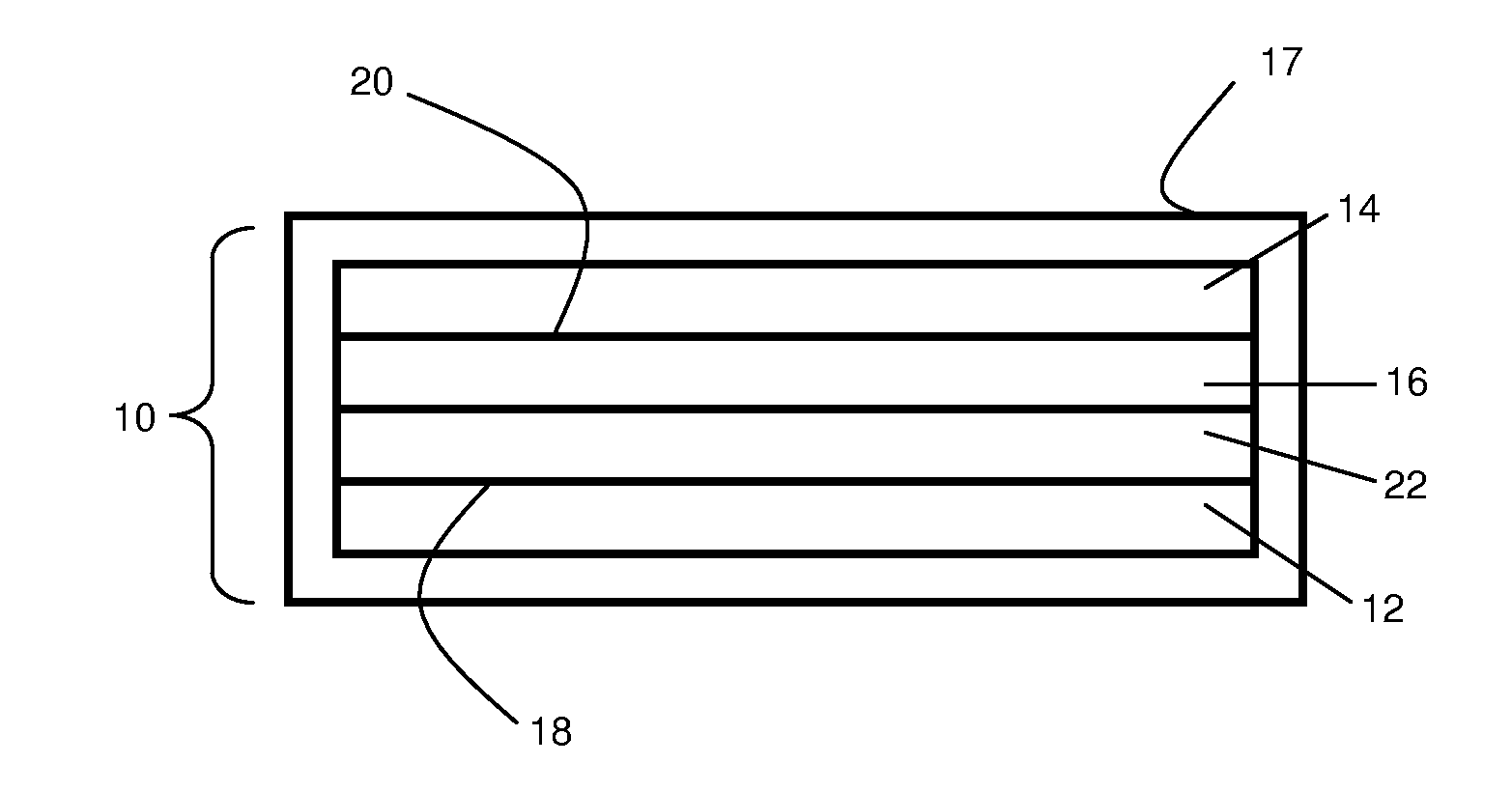
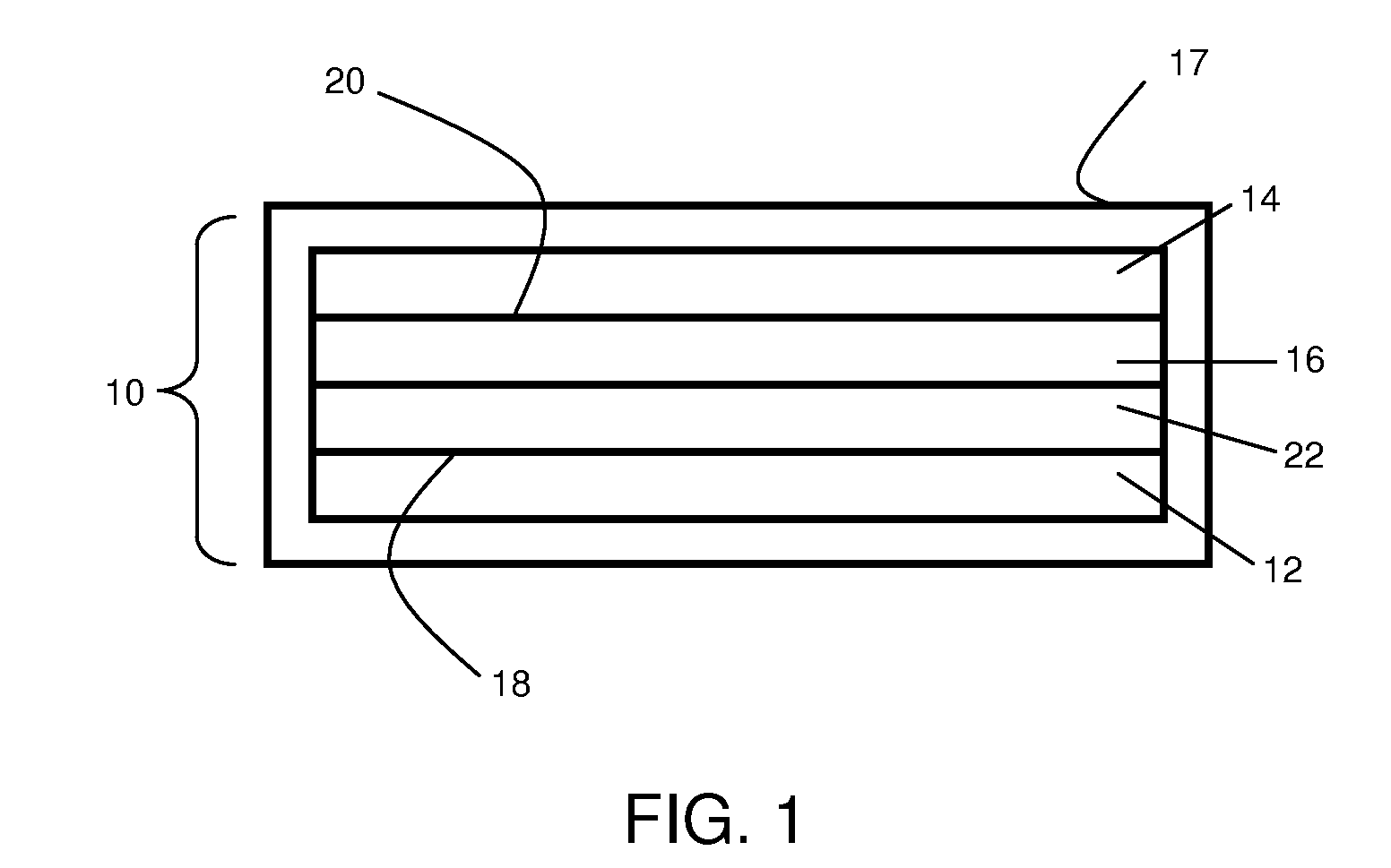
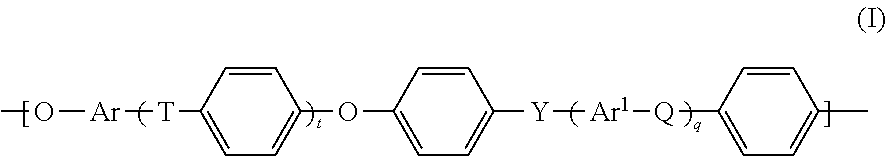
Electrolyte materials for use in electrochemical cells
a technology of electrochemical cells and electrochemical materials, applied in the field of electrochemical cells, can solve the problems of limited capacity, incomplete destruction of batteries, and many technical problems that have not yet been solved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0171]This example outlines, according to one set of embodiments, the manufacture of an inventive electrochemical cell EC.1
[0172]A slurry was prepared from a solution of polyethersulfone (C.1) (10% wt.) and filler (G.1) (10% wt.) in diethyleneglycol dimethyl ether was coated onto vacuum deposited lithium (VDL) on a web coater. The weight ratio of polysulfone / silica was 7:3. The gel layer was dried in a gel coater oven at 65-80° C. An anode (B.1) with a dry gel layer was obtained. The resulting thickness of the dry gel layer was 7 μm. A triple bi-cell with above anodes (B.1), separators (I.1) and cathodes (A.1) was assembled and filled with electrolyte 1. Inventive electrochemical cell EC.1 was obtained. Inventive electrochemical cell EC.1 displayed 1015 mAh / g sulfur specific capacity on the 5th discharge. Inventive electrochemical cell EC.1 was cycled for 10 cycles and went for a safety test. The inventive electrochemical cell EC.1 at fully charged conditions was ramped at 5° C. / min...
example 2
[0174]This example describes, according to some embodiments, the manufacture of an inventive electrochemical cell EC.3.
[0175]A slurry prepared from a solution of polyethersulfone (C.1) (10% wt.) and filler (G.2) (10% wt.) in diethyleneglycol dimethyl ether was coated onto VDL on a web coater. The weight ratio of polysulfone / silica was 7:3. The gel layer was dried in a gel coater oven at 65-80° C. An anode (B.3) with a dry gel layer was obtained. The resulting thickness of the dry gel layer was 7 μm. A triple bi-cell with above anodes (B.3), separators (I.1) and cathodes (A.1) was assembled and filled with electrolyte 1. Inventive electrochemical cell EC.3 was obtained. Inventive electrochemical cell EC.3 displayed 1025 mAh / g specific capacity on the 5th discharge. Inventive electrochemical cell EC.3 was cycled for 10 cycles and went for the safety test. Inventive electrochemical cell EC.3 was ramped to 230° C. without going into thermal runaway.
example 3
[0176]This example describes the manufacture of an inventive electrochemical cell EC.4, according to one set of embodiments.
[0177]A slurry prepared from a solution of polyethersulfone (C.1) (10% wt.) and filler (G.3) (10% wt.) in diethyleneglycol dimethyl ether was coated onto VDL on a web coater. The weight ratio of polysulfone / silica was 1:1. An anode (B.4) with a dry gel layer was obtained. The gel layer was dried in a gel coater oven at 65-80° C. An anode (B.4) with a dry gel layer was obtained. The resulting thickness of the dry gel layer was 7 μm. A triple bi-cell (EC.4) with above anodes (B.3), separators (I.2) and cathodes (A.1) was assembled and filled with electrolyte 1. Inventive electrochemical cell EC.4 was obtained. Inventive electrochemical cell EC.4 displayed 1019 mAh / g specific capacity on the 5th discharge.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com