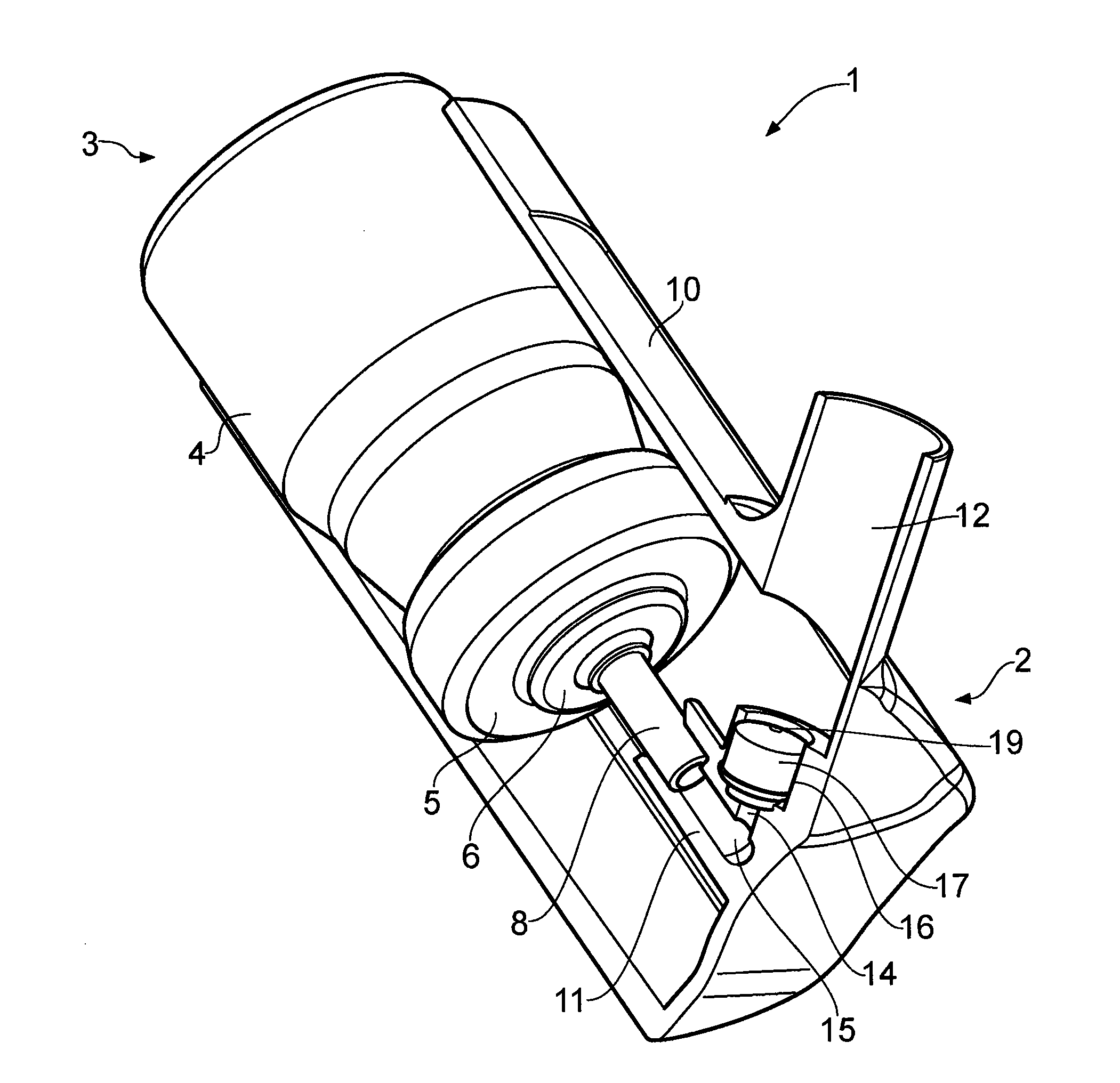
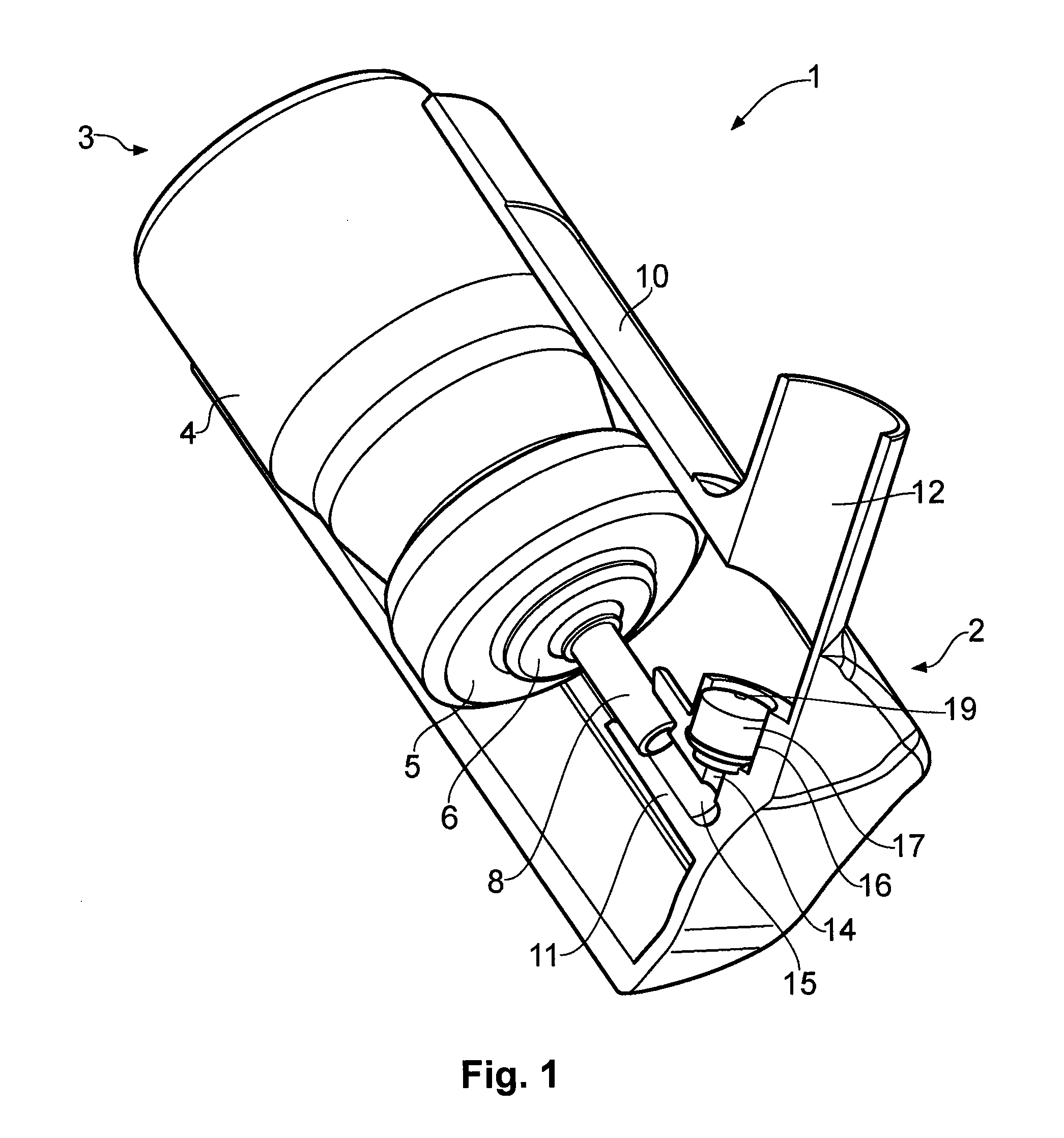
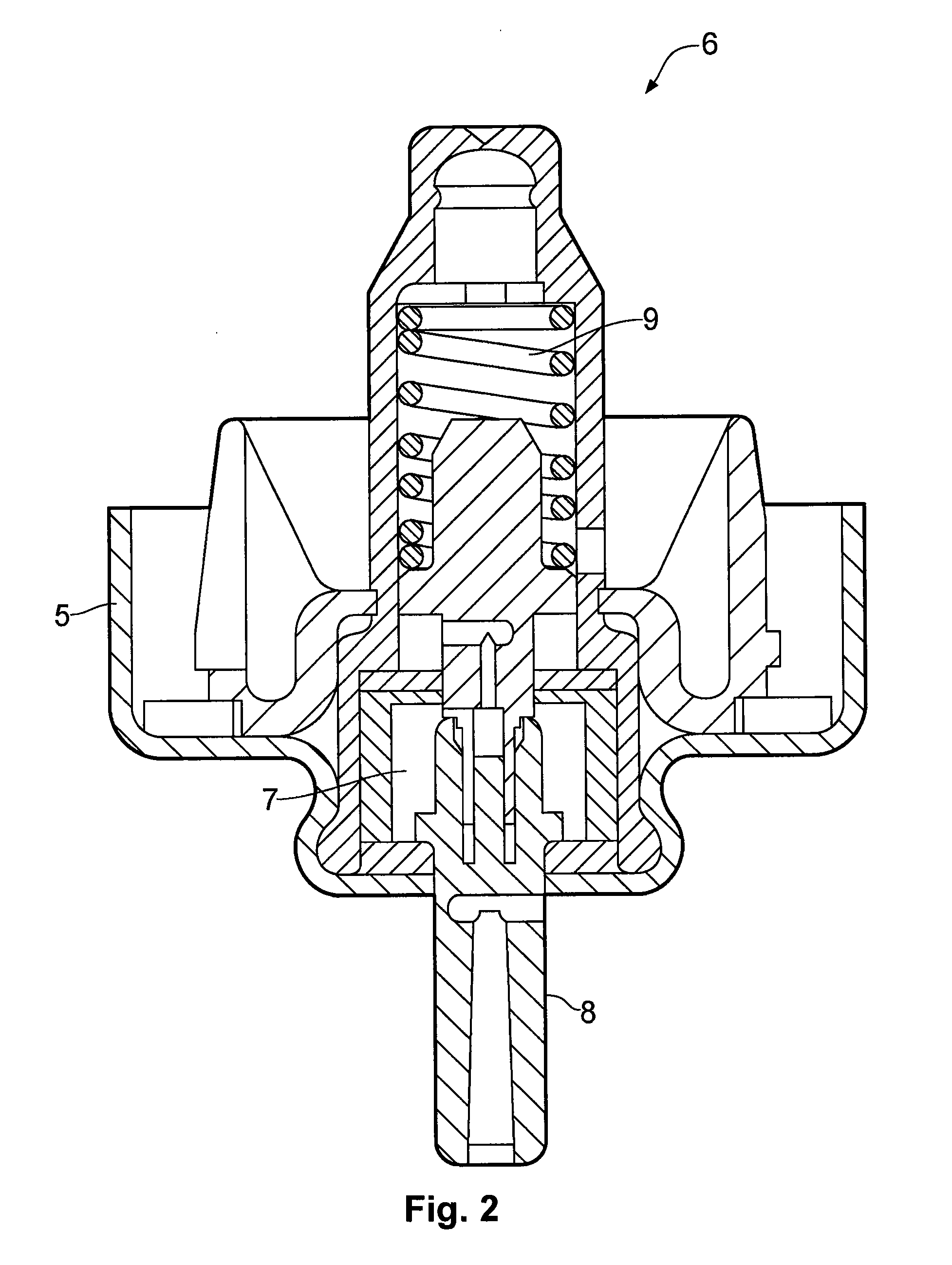
Nasal spray device
a spray device and spray tube technology, applied in the direction of aerosol delivery, immunologic disorders, drug compositions, etc., can solve the problems of difficult repeatability, large actuation force of manual pumps, young and elderly,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052]Spray force values for a nasal spray device according to the present invention were measured using a variety of actuation velocities and accelerations. The device tested was of the type shown in FIGS. 1 and 3 and configured with a nose piece having an inner diameter of 8.2 mm. The stem block insert had the shape generally shown in FIG. 4. The orifice size is 0.4 mm and insert length is 10 mm. The device was loaded with a HFA aerosol canister configured to provide an 80 μg dose (ex-valve) of beclomethasone dipropionate. The solution formulation consisted of the beclomethasone dipropionate as the active ingredient, together with ethanol 4.8 mg per actuation as a co-solvent and P134a 55.1 mg per actuation as a propellant. Spray force values for three commercially available manual pump-type nasal spray devices were also measured using the same variety of actuation velocities and accelerations for comparison purposes. Details of the devices tested are summarised in Table 1.
TABLE 1D...
examples 2-5
[0081]Further testing was carried out on the test devices of the type shown in FIGS. 1 and 3 having different stem block inserts. The devices were each configured with a nose piece having an inner diameter of 7.2 mm. The stem block insert of each device had the shape generally shown in FIG. 4, with the dimensions provided in Table 6. The orifice size is 0.4 mm, the insert length of 10 mm, a land length of 0.65 mm, and a tip diameter of 6.4 mm. The device was loaded with an HFA aerosol canister configured to provide a 100 μg dose (ex-valve) of beclomethasone dipropionate. The solution formulation consisted of the beclomethasone dipropionate as the active ingredient, together with ethanol 4.8 mg per actuation as a co-solvent and P134a 55.1 mg per actuation as a propellant.
TABLE 6DevicesDischarge orificeInsertExample no.diameter (mm)length (mm)Example 20.225Example 30.2210Example 40.45Example 50.410Comparative Example 40.75Comparative Example 50.710
[0082]The nasal spray devices were te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| spray force | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com