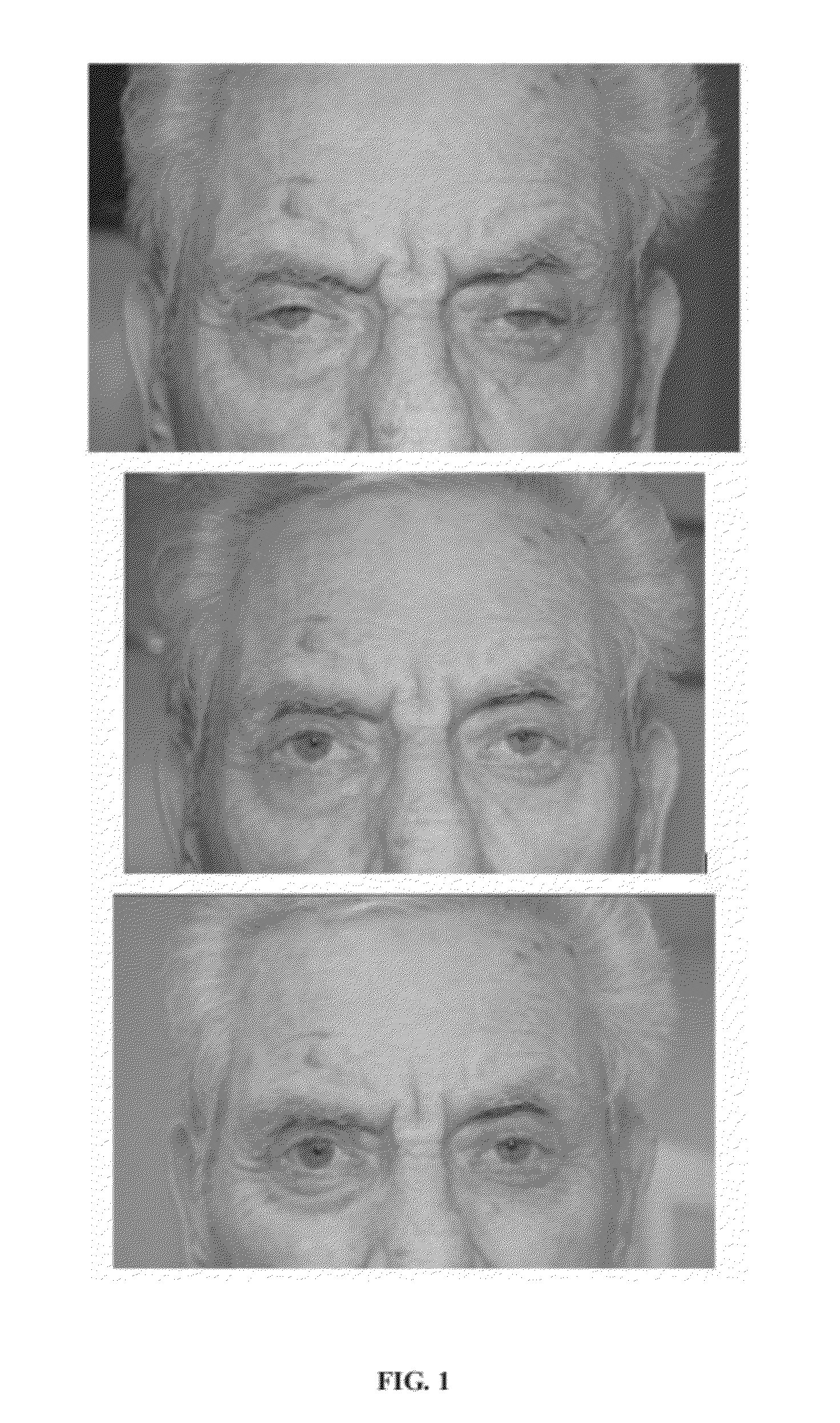
Compositions and Methods for Non-Surgical Treatment of Ptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Non-Blinded, Uncontrolled Study with 0.1% Oxymetazoline in Subjects with Unilateral Ptosis
[0124]In this example, a single drop of 0.1% oxymetazoline solution was placed in the affected eye of each of five adult human subjects with unilateral ptosis. Palpebral fissure was measured at baseline (pre-treatment), then at 30 minutes and at 4 hours following treatment. Measurements were taken with a fine point metric ruler, measuring (in mm) the central diameter of the palpebral fissure (i.e., sagitally across the center of the pupil). Results are shown in Table 1. “OD” refers to right eye; “OS” refers to left eye. “Rx” refers to which eye was treated. “% Δ (4 hr)” is the percent change 4 hours following treatment. All measurements are reported in mm. As shown in Table 1, 0.1% oxymetazoline vertically widened the palpebral fissure in 5 / 5 (100%) of subjects, and this effect lasted at least 4 hours. The mean increase from baseline, 4 hours following treatment, was 2 mm or 31.4%.
TABLE 1Subjec...
example 2
Double-Blind, Randomized, Controlled Study with 0.1% Oxymetazoline v. Vehicle Alone in Normal Subjects
[0125]In this example, a single drop of 0.1% oxymetazoline solution was randomly assigned to be placed in one eye of each of five normal adult human subjects; a single drop of vehicle alone (negative control) was placed in the other eye of each subject. Palpebral fissure was measured at baseline (pre-treatment), then at 1 hour and at 4 hours following treatment. Measurements were taken with a fine point metric ruler, measuring (in mm) the central diameter of the palpebral fissure (i.e., sagitally across the center of the pupil). Results are shown in Table 2. “OD” refers to right eye; “OS” refers to left eye. “Rx” refers to treatment. “Oxy” refers to oxymetazoline; “V” refers to vehicle. “% Δ (4 hr)” is the percent change 4 hours following treatment. All measurements are reported in mm. As shown in Table 2, 0.1% oxymetazoline vertically widened the palpebral fissure in 5 / 5 (100%) of ...
example 3
Double-Blind, Randomized, Controlled Study with 0.1% Oxymetazoline v. Visine® L.R.® in Normal Subjects
[0126]In this example, a single drop of 0.1% oxymetazoline solution was randomly assigned to be placed in one eye of each of ten normal adult human subjects; a single drop of 0.025% oxymetazoline (Visine® L.R.®, positive control) was placed in the other eye of each subject. Palpebral fissure was measured at baseline (pre-treatment), then at minutes and at 3 hours following treatment. Measurements were taken with a fine point metric ruler, measuring (in mm) the central diameter of the palpebral fissure (i.e., sagitally across the center of the pupil). Results are shown in Table 3. “OD” refers to right eye; “OS” refers to left eye. “Rx” refers to treatment. “Oxy” refers to 0.1% oxymetazoline; “Vis” refers to Visine® L.R.® (0.025% oxymetazoline). “% Δ (3 hr)” is the percent change 3 hours following treatment. All measurements are reported in mm. As shown in Table 3, 0.1% oxymetazoline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com