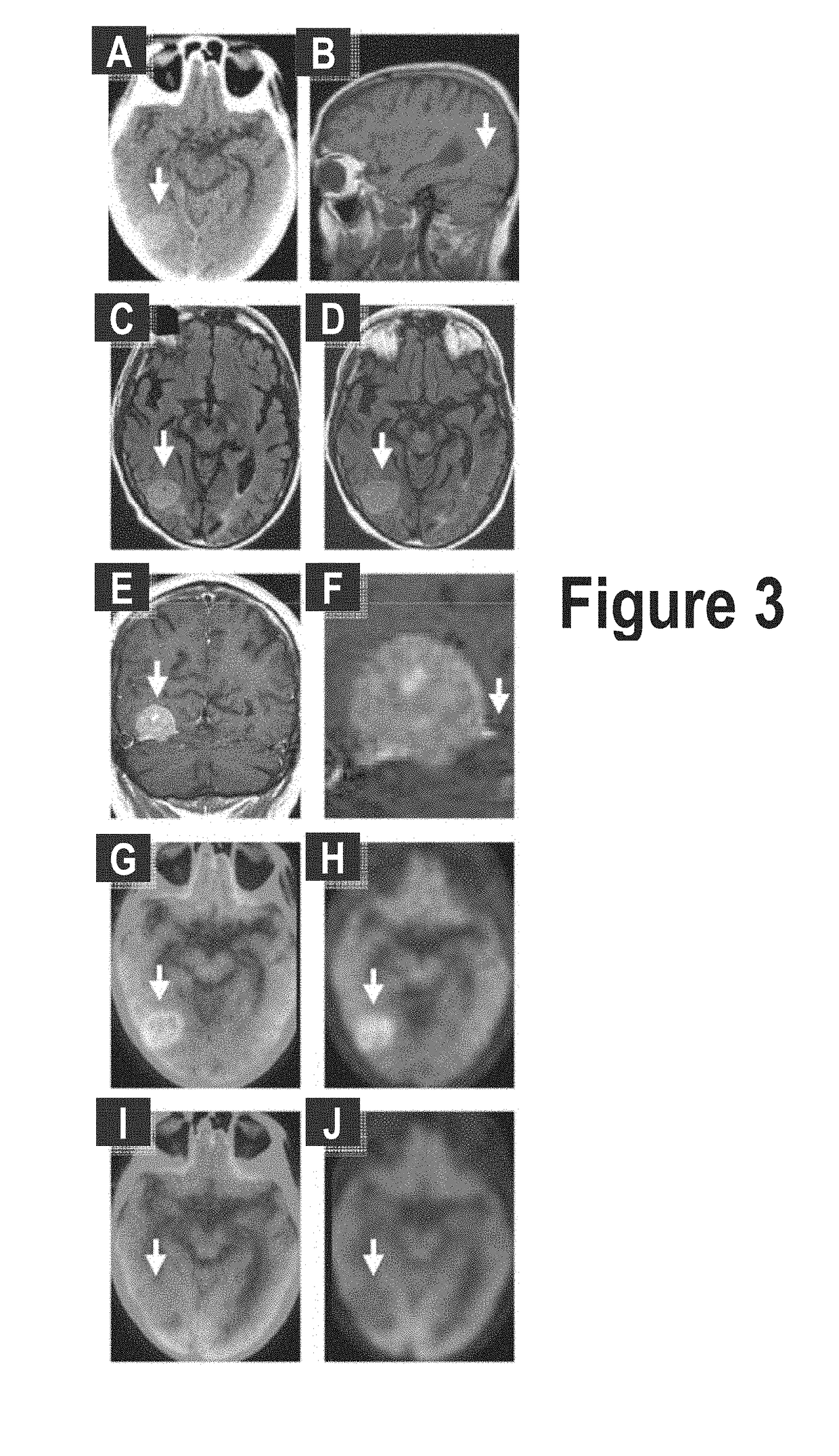
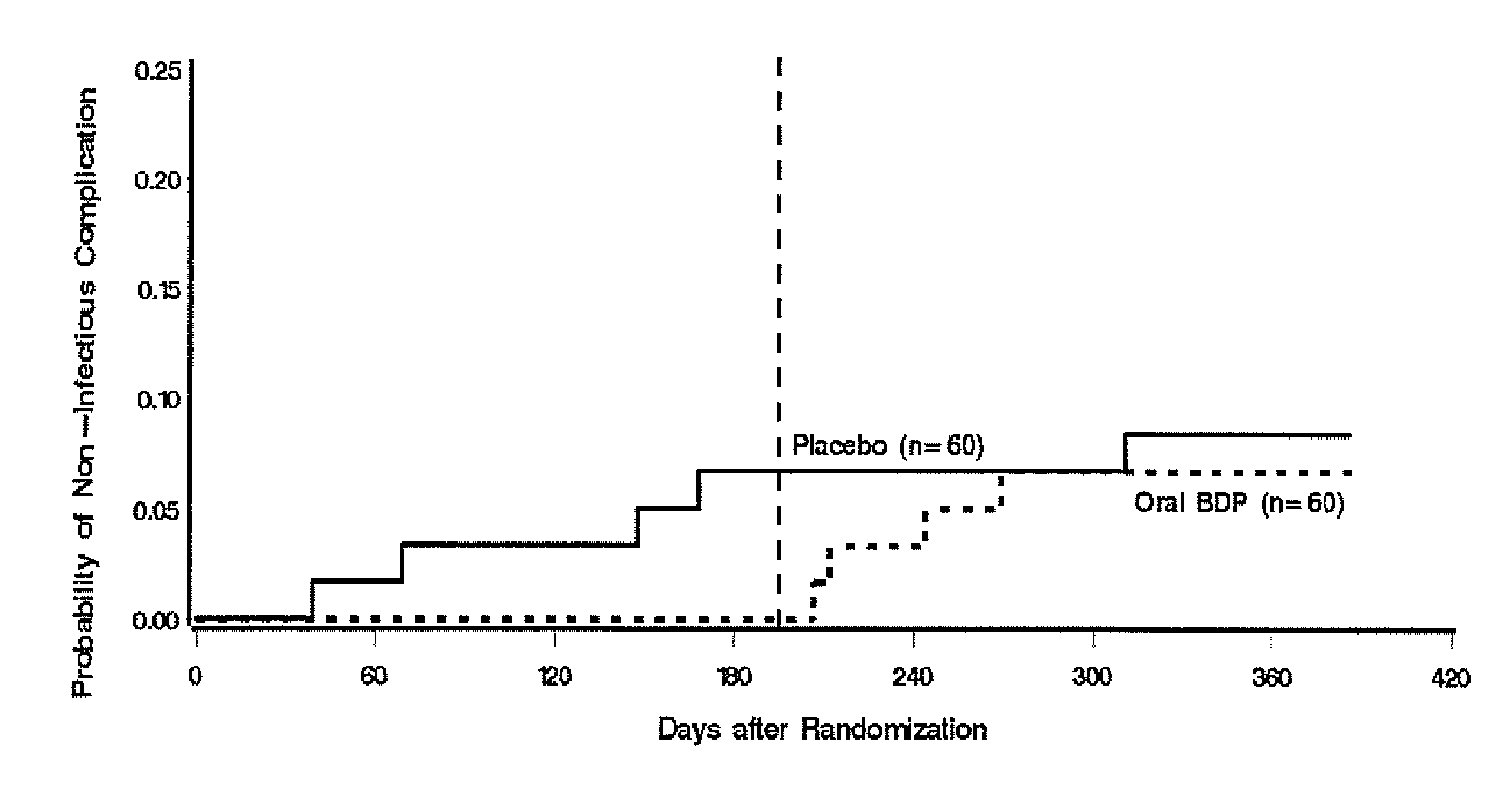
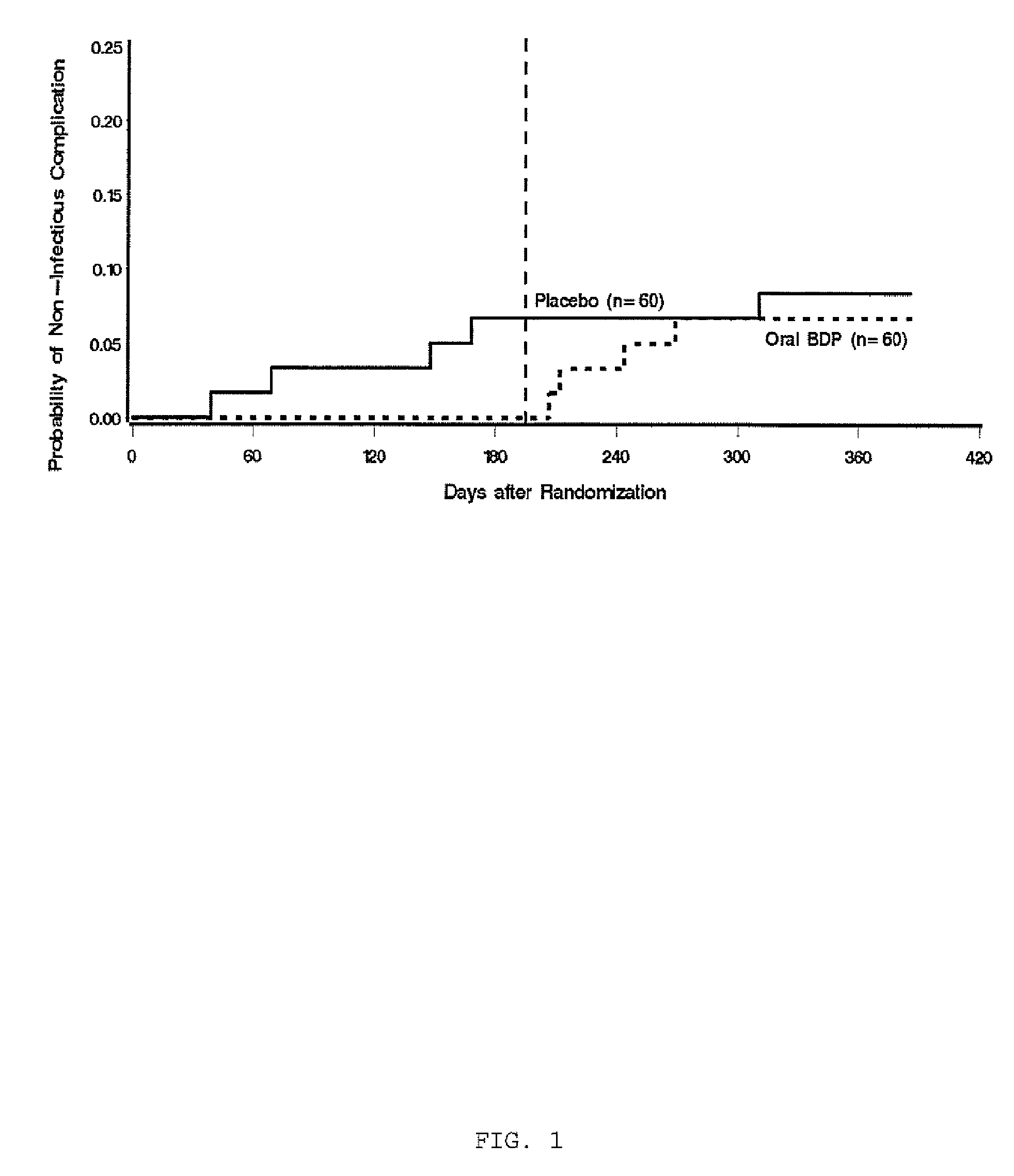
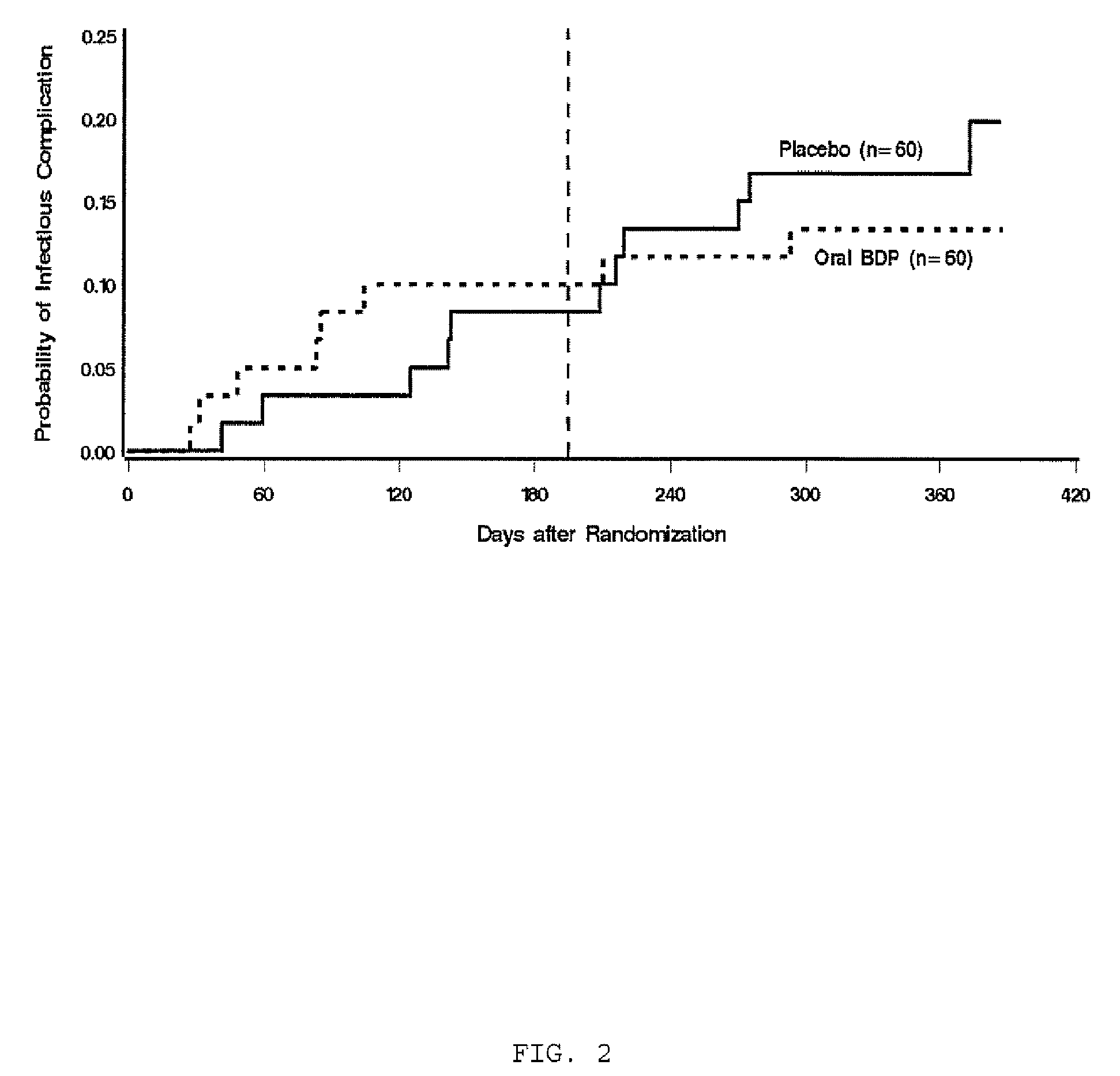
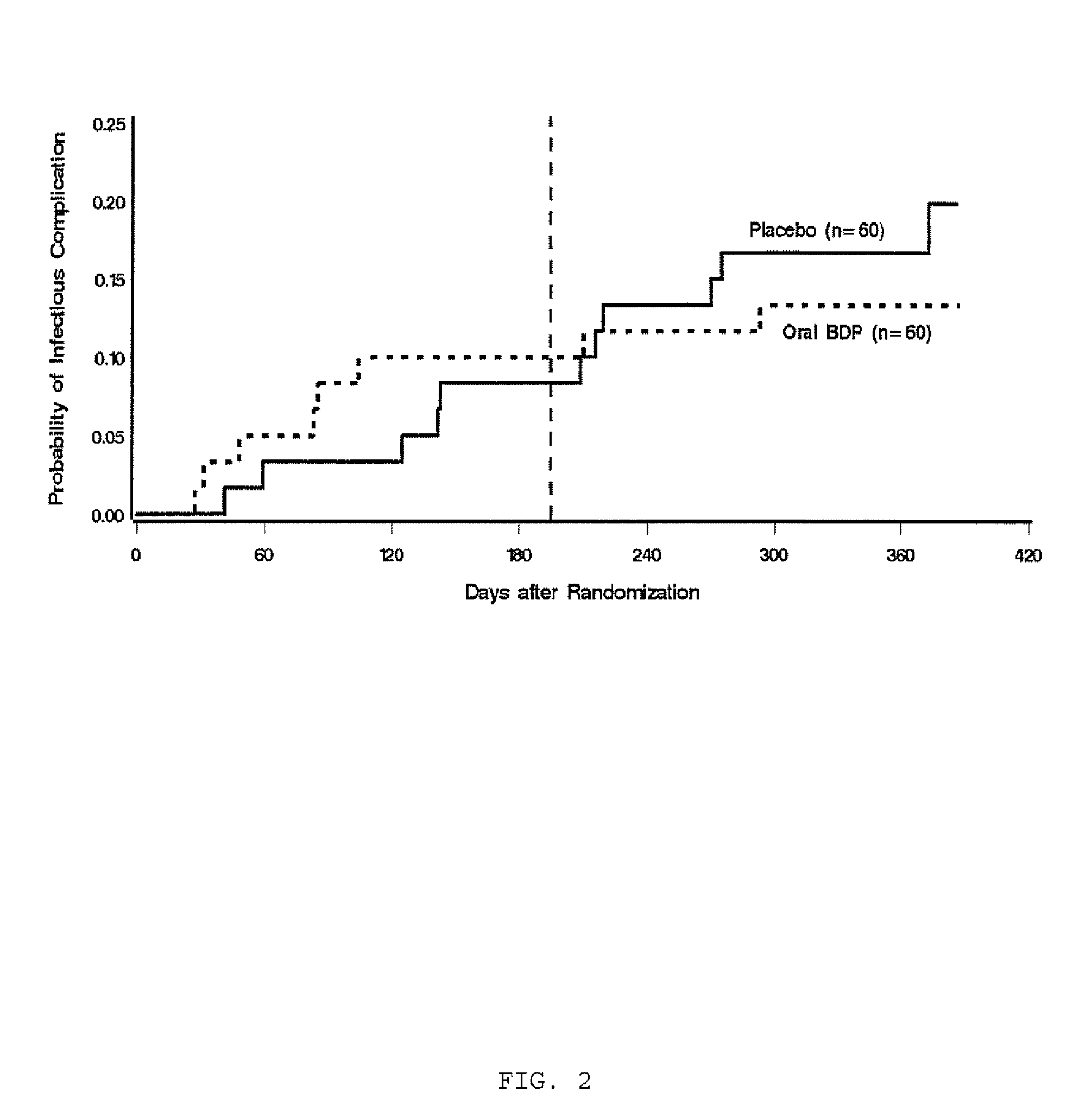
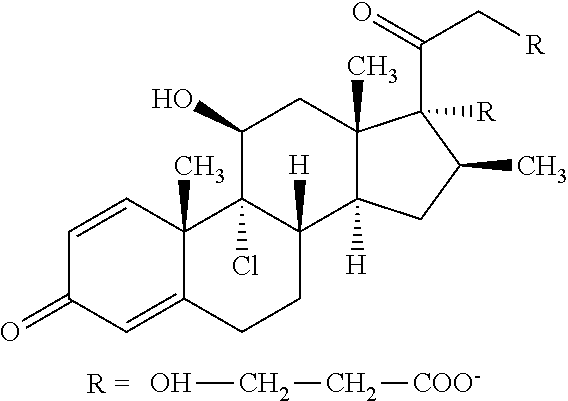
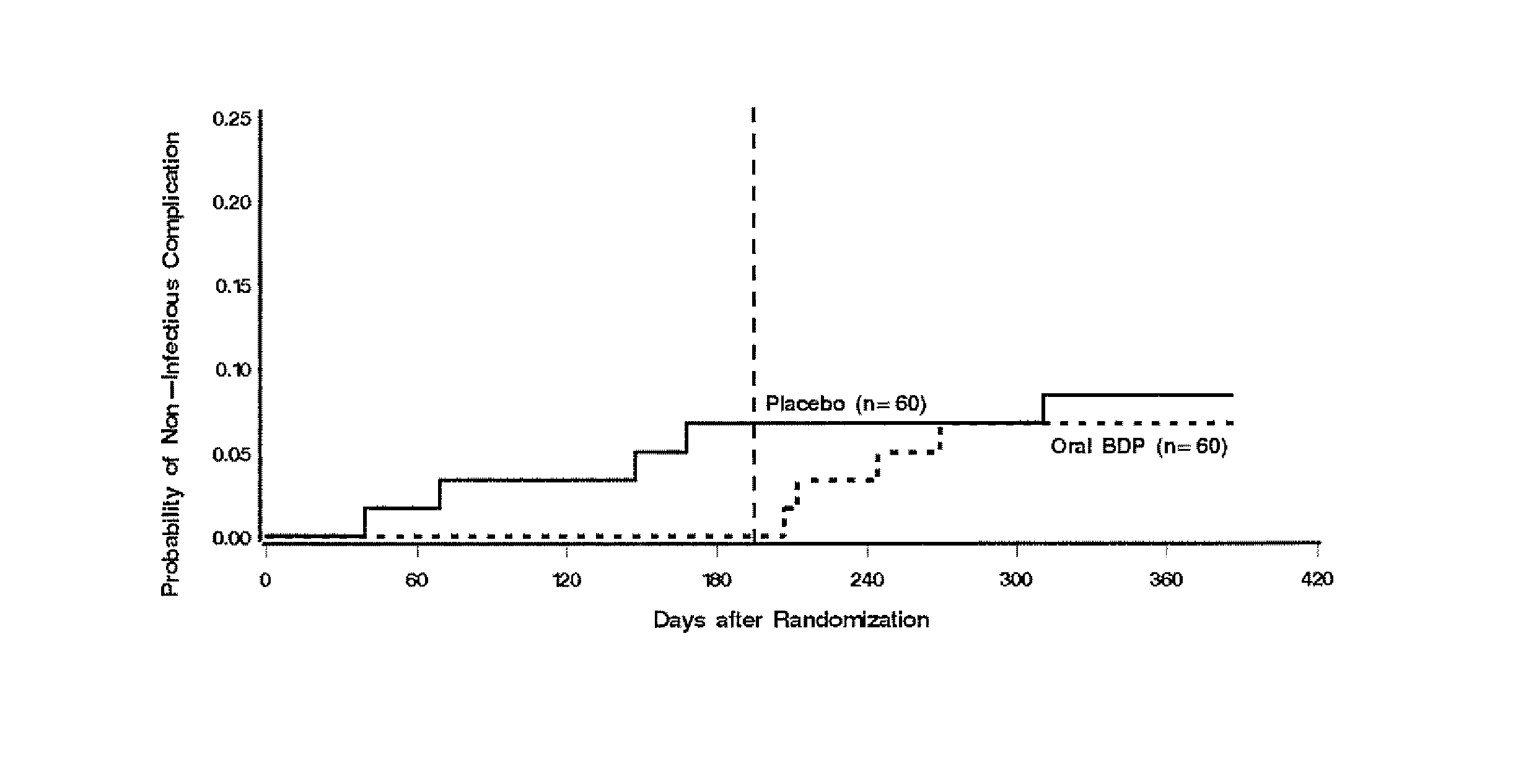
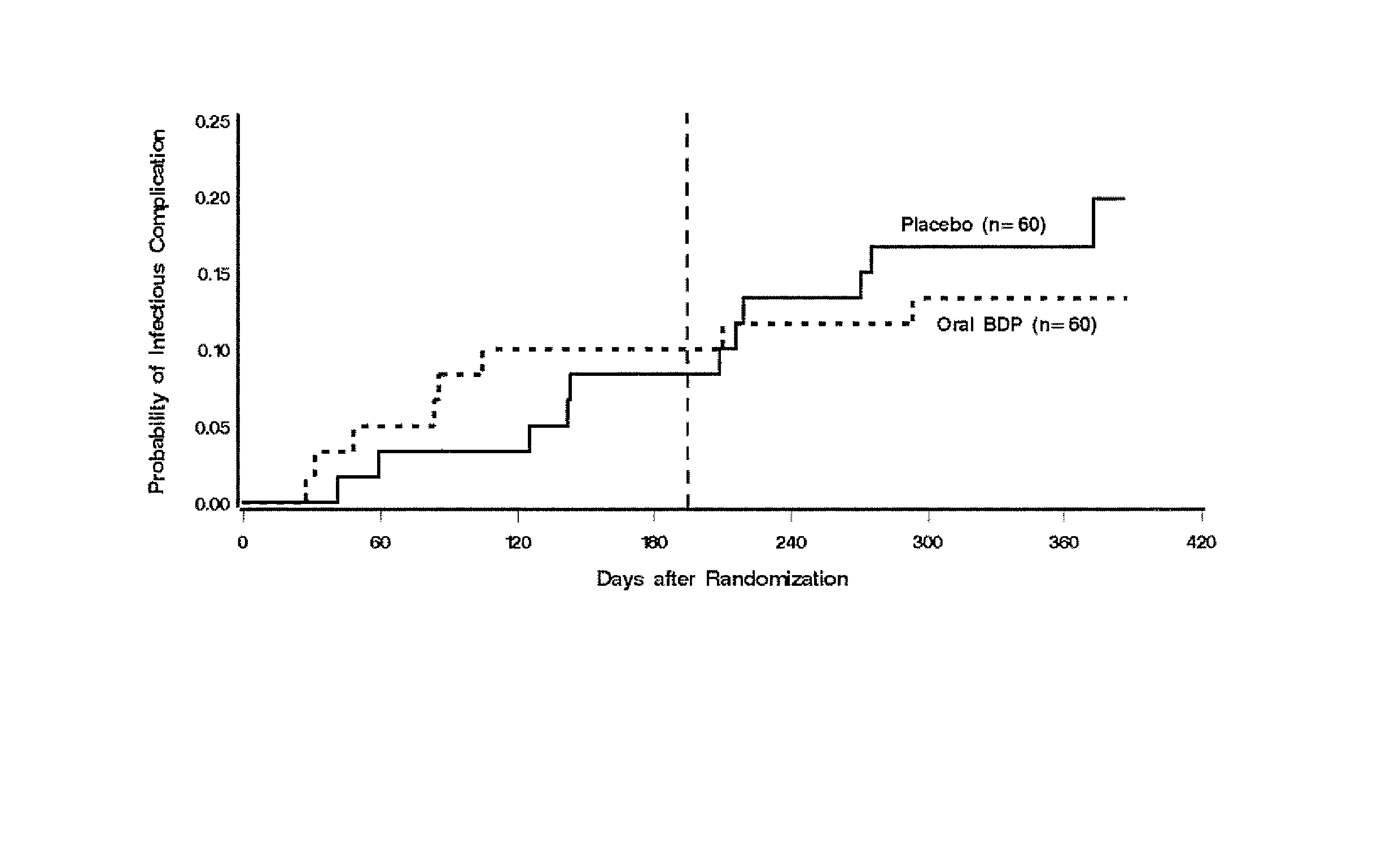
Patents
Literature
33results about How to "Side effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
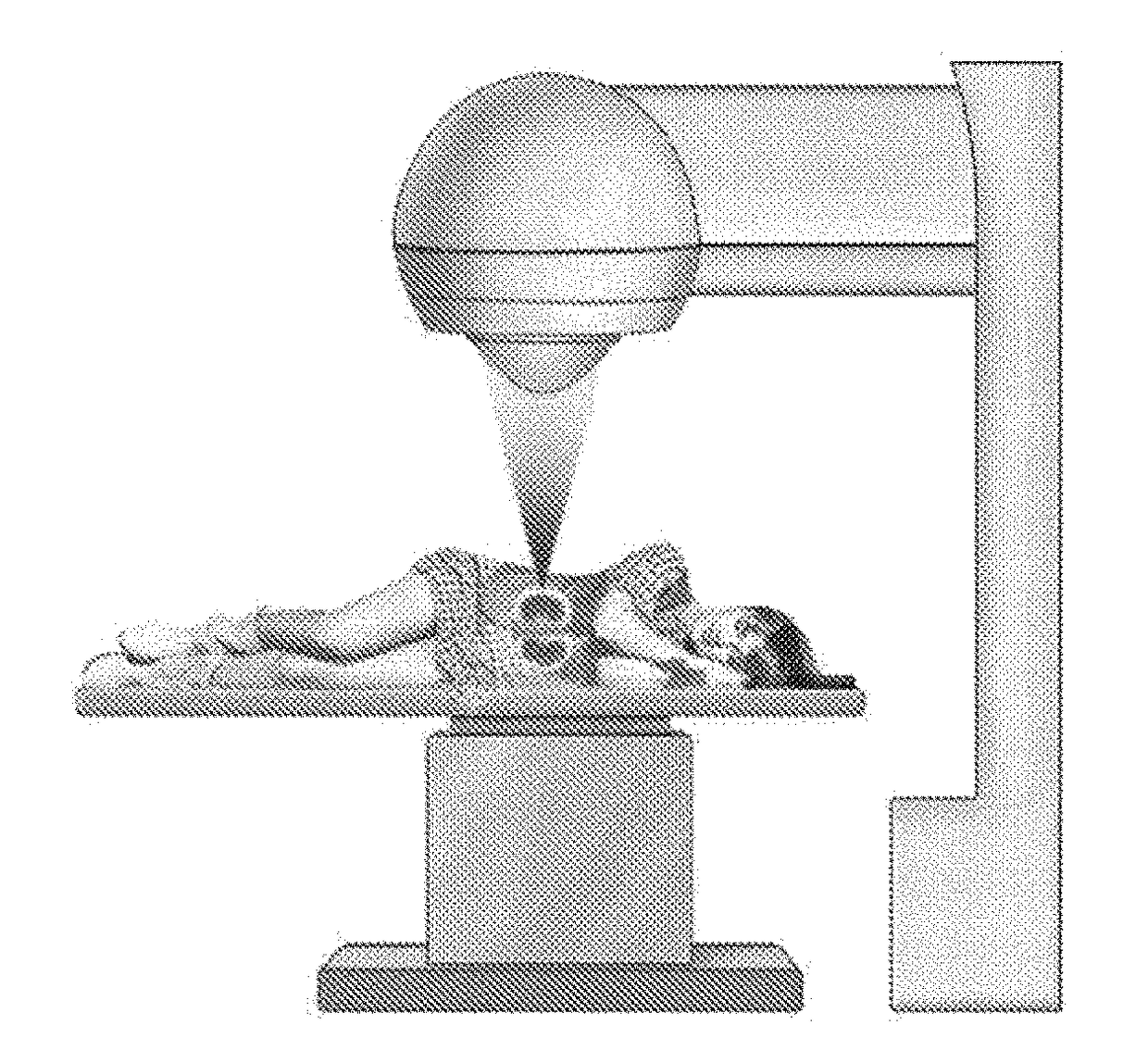
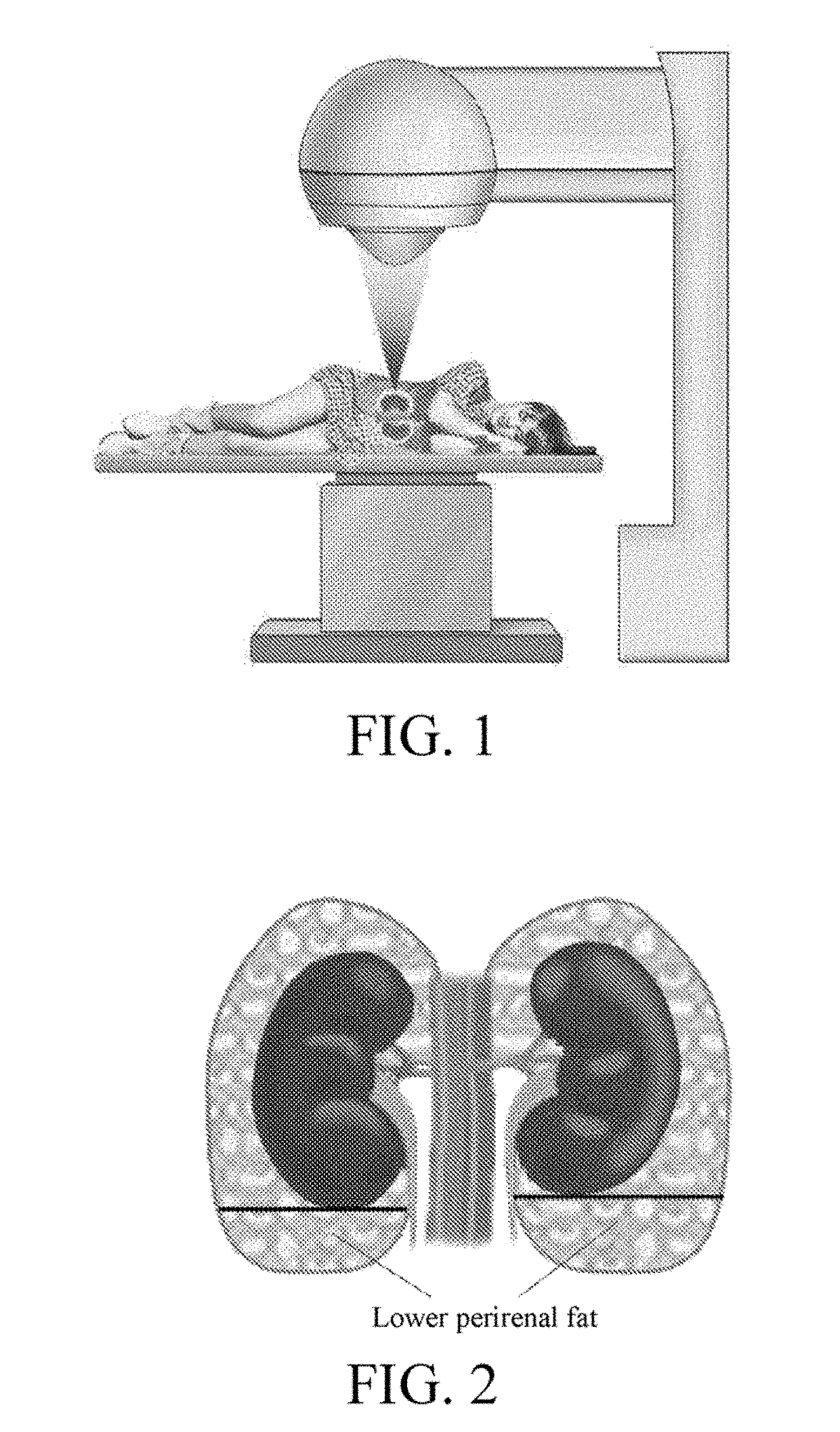
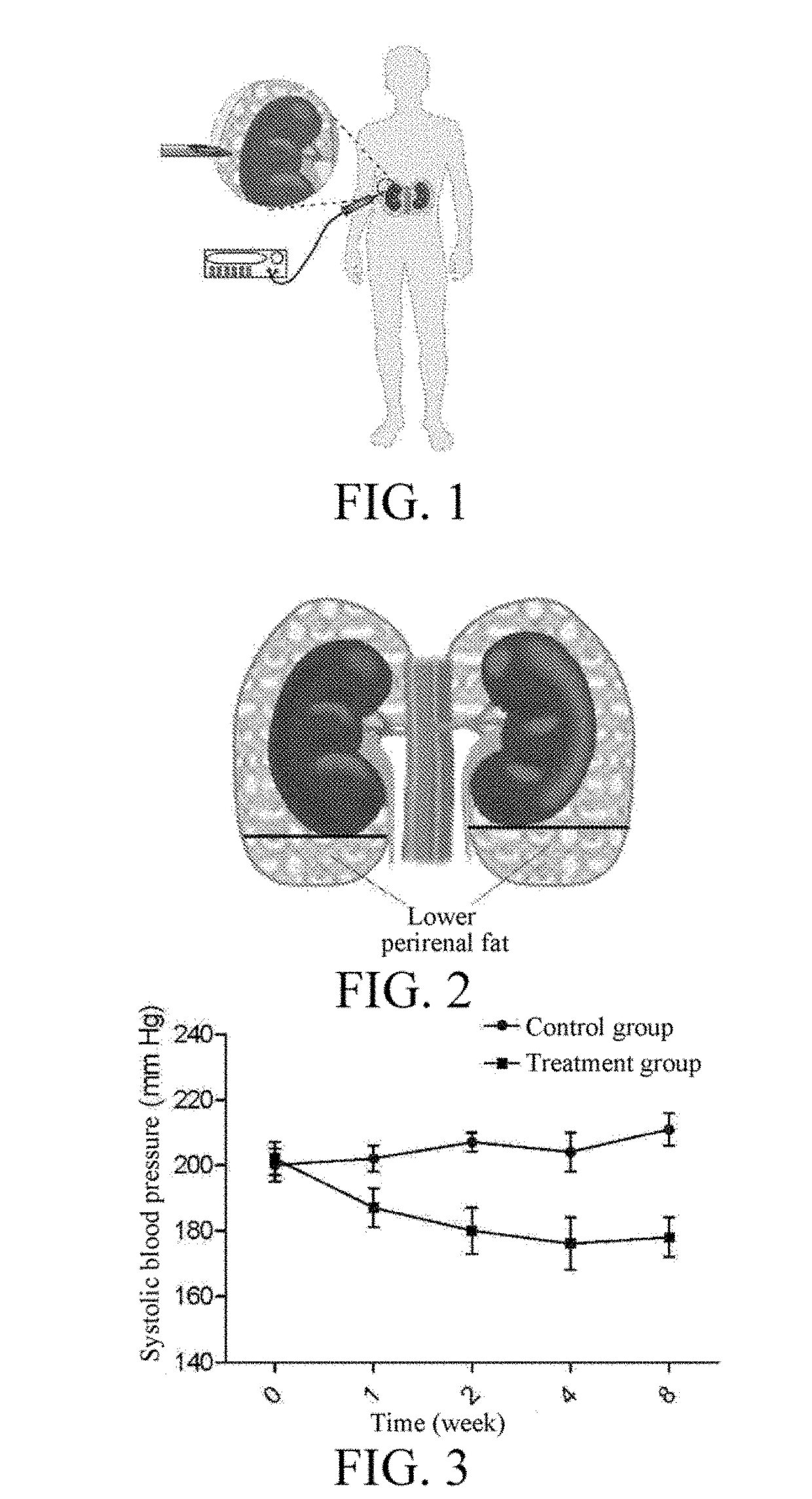
Application of high-intensity focused ultrasound system to treatment of essential hypertension
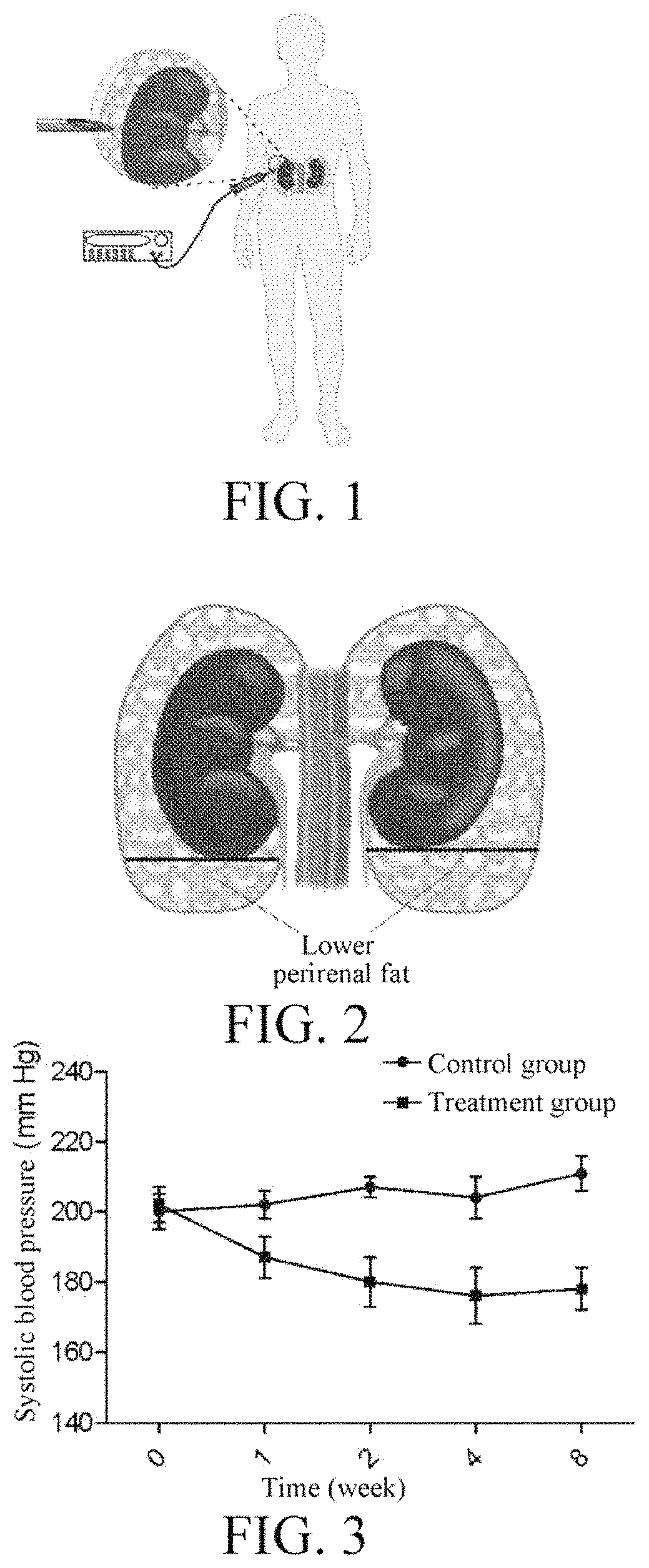
InactiveUS20180154184A1Blood pressure level is reducedSmall doseUltrasound therapySurgical instrument detailsNervous systemWhole body
An application of a high-intensity focused ultrasound system to treatment of essential hypertension. A treatment process includes: conducting ultrasonic positioning measurement on both sides of perirenal fat tissue of a patient, setting a therapeutic window and parameters of the system, and setting corresponding power parameter of the system, so as to make local temperature of the tissue during treatment reach 40-70° C., wherein treatment portions are both sides, and treatment scope is one third to all of the whole tissue; starting the system to treat one side of the tissue according to the set parameters and then the other. By treating a secondary center for regulating the activity of a whole-body sympathetic system, the activity of the whole-body sympathetic system can be reduced, so that the blood pressure level of the patient can be reduced, and fewer kinds and smaller dosage of antihypertensive drugs can be taken or ceased.
Owner:NANJING GUANGCI MEDICAL TECH
Method for preparing bioabsorbable organic/inorganic composition for bone fixation devices and itself prepared thereby
InactiveUS20040253290A1Good dispersionEasy to manufactureSurgical adhesivesProsthesisSide effectCalcium phosphorus
A high-strength, biodegradable, organic polymer / inorganic particle composite material for bone fixation, which is prepared by mixing and dispersing a biocompatible, inorganic fine or ultrafine particle in an organic monomer and then polymerizing the organic monomer and thus exhibits remarkably improved mechanical strength, and also to a high-strength, biodegradable, organic polymer / inorganic particle composite material prepared thereby is disclosed. More particularly, the a high-strength, biodegradable, organic polymer / inorganic particle composite material for bone fixation is prepared by mixing and dispersing a calcium phosphorus compound or a calcium aluminate compound in a biodegradable organic monomer, at the amount of 0.5 to 60% by weight; and polymerizing the biodegradable organic monomer; and then forming the polymerized material into a desired shape. The synergistic, reinforcing effect of the inorganic fine particle is increased, so that the high-strength material for bone fixation can be prepared. Also, the biocompatible fine particle is used, so that a long-term side effect can be reduced.
Owner:CHEM & MEDICAL RES
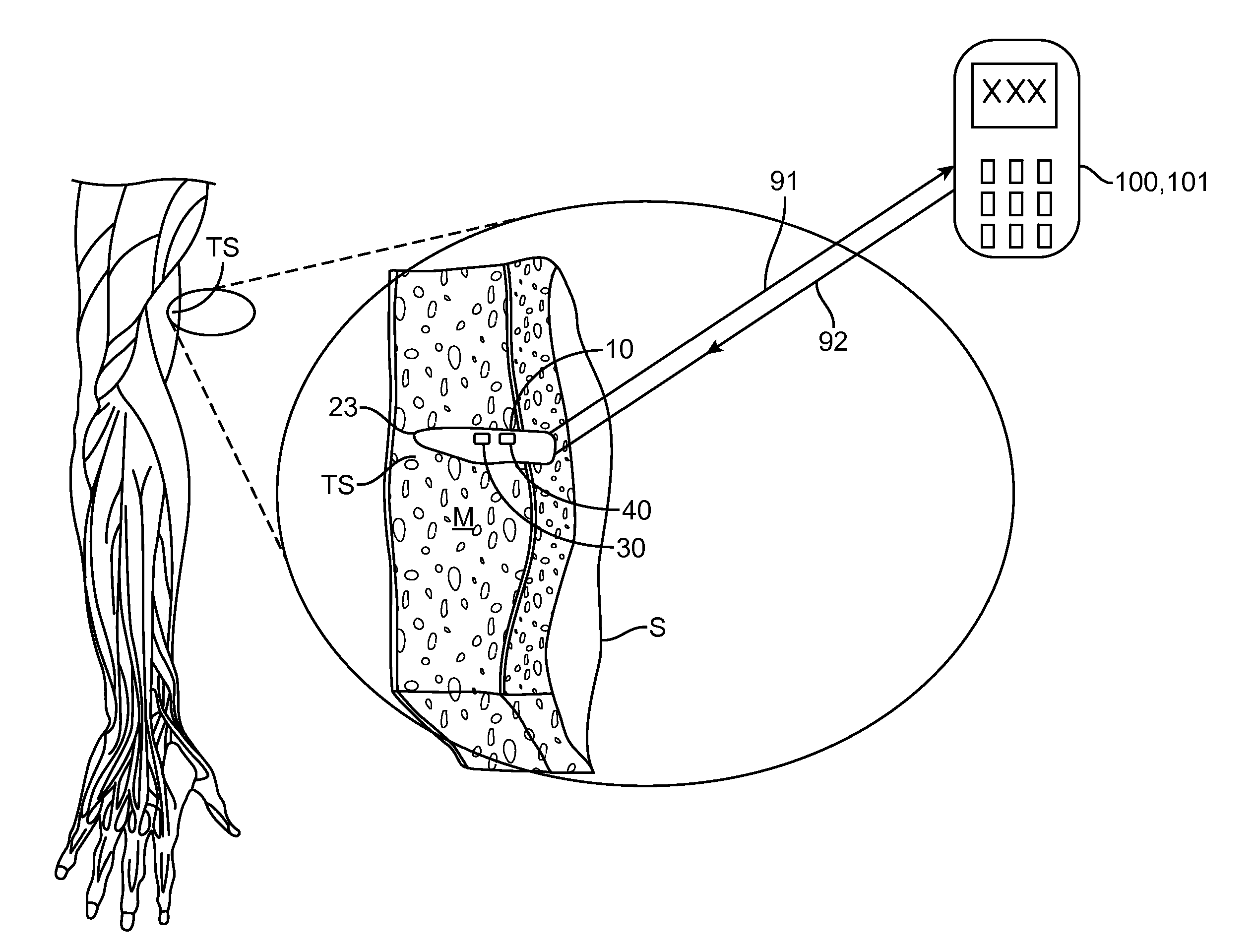
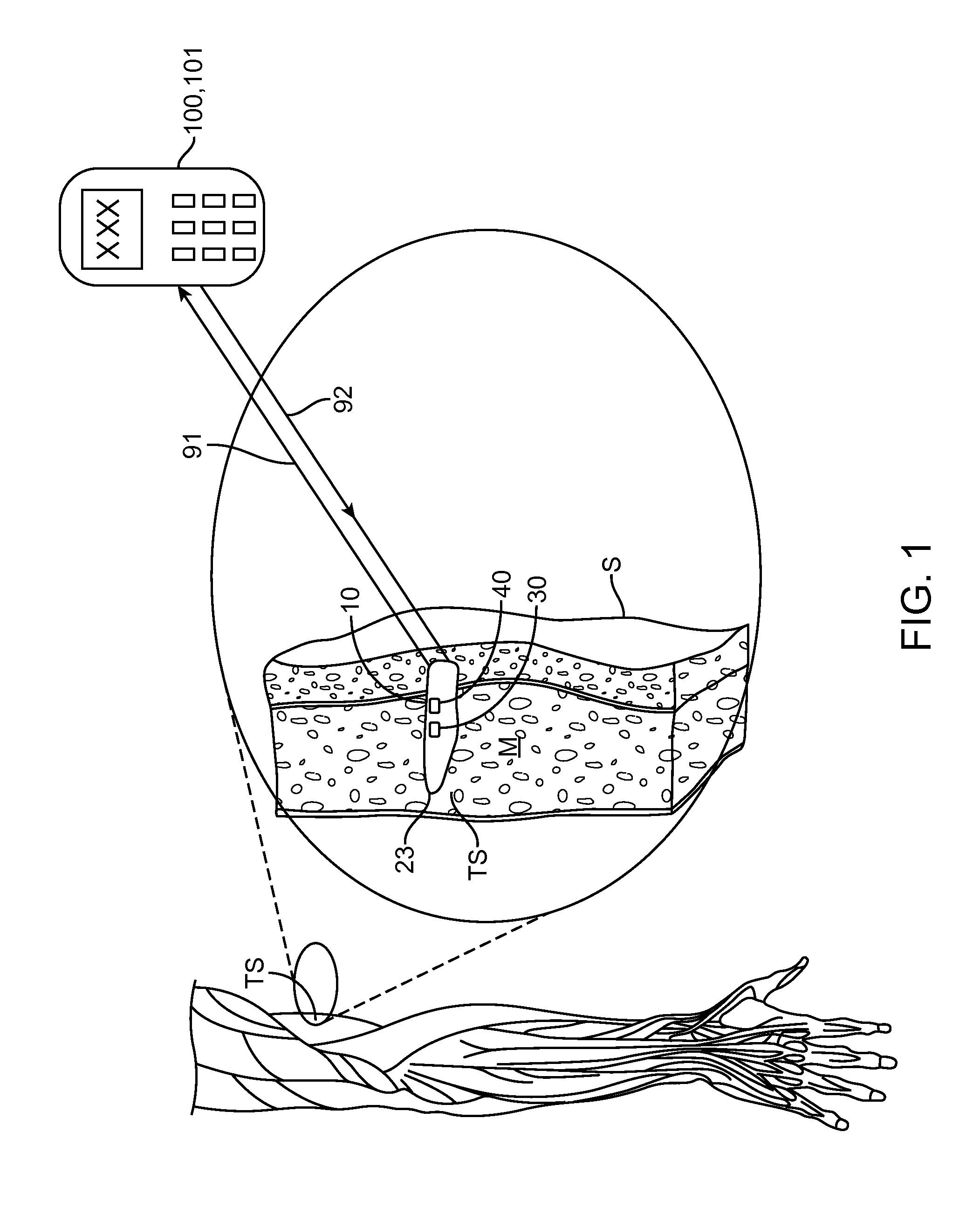
Implantable oximetric measurement apparatus and method of use
InactiveUS20130289372A1Easy to disassembleRelieve painCatheterDiagnostic recording/measuringLength waveUltimate tensile strength
Embodiments provide an apparatus, system, kit and method for in vivo measurement of blood oxygen saturation (BAS). One embodiment provides an implantable apparatus for measuring BAS comprising a housing, emitter, detector, processor and power source. The housing is configured to be injected through a tissue penetrating device into a target tissue site (TS). The emitter is configured to emit light into the TS to measure BAS, the emitted light having at least one wavelength (LOW) whose absorbance is related to a BAS. The detector is configured to receive light reflected from the TS, detect light at the LOW and generate a detector output signal (DOS) responsive to an intensity of the detected light. The processor is operably coupled to the detector and emitter to send signals to the emitter to emit light and receive the DOS and includes logic for calculating a BAS and generate a signal encoding the BAS.
Owner:INCUBE LABS
Prevention and treatment of cardiac conditions
InactiveUS20080275036A1Efficient treatment methodMinimizing undesirable side effectOrganic active ingredientsBiocideHeart diseaseEndocrinology
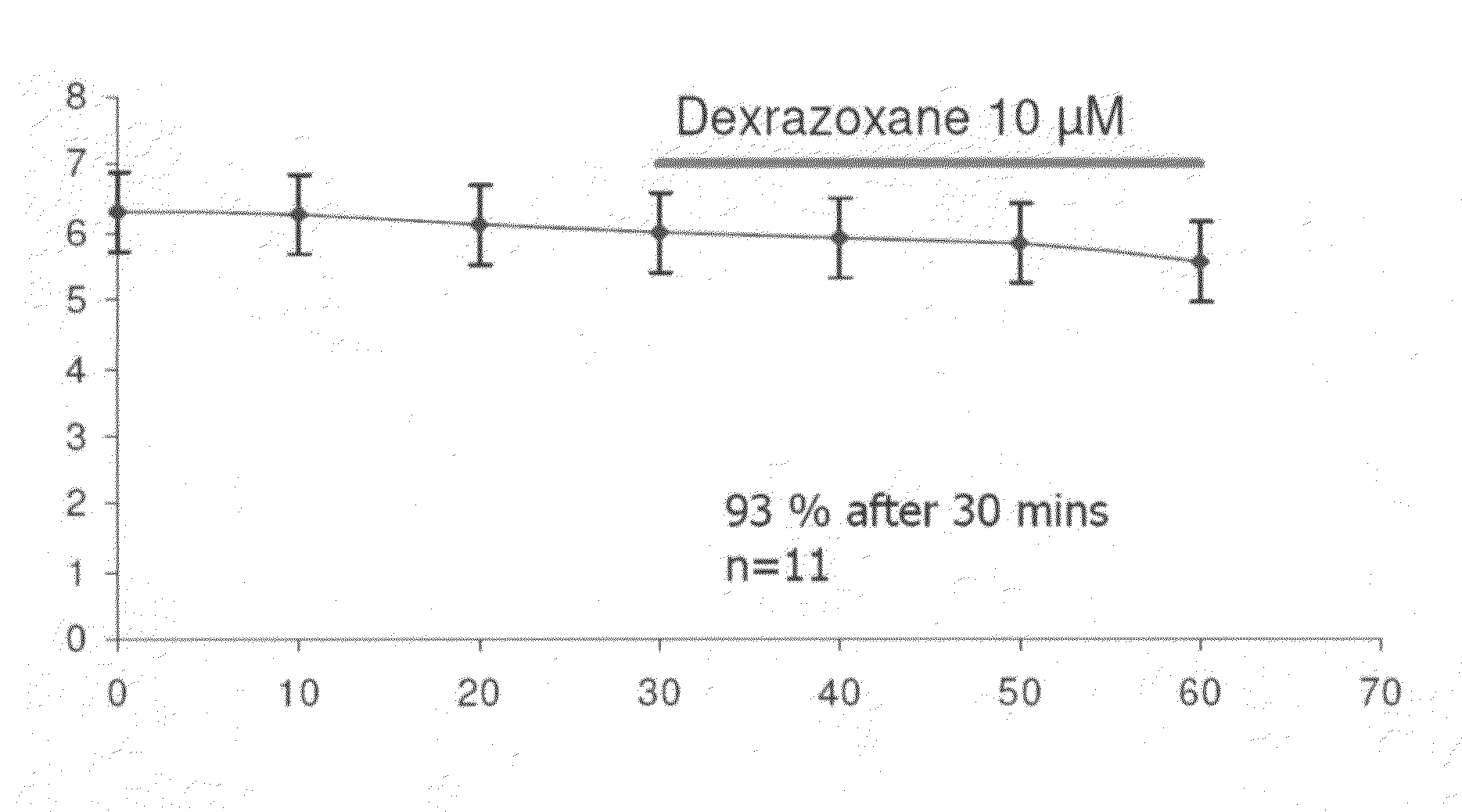
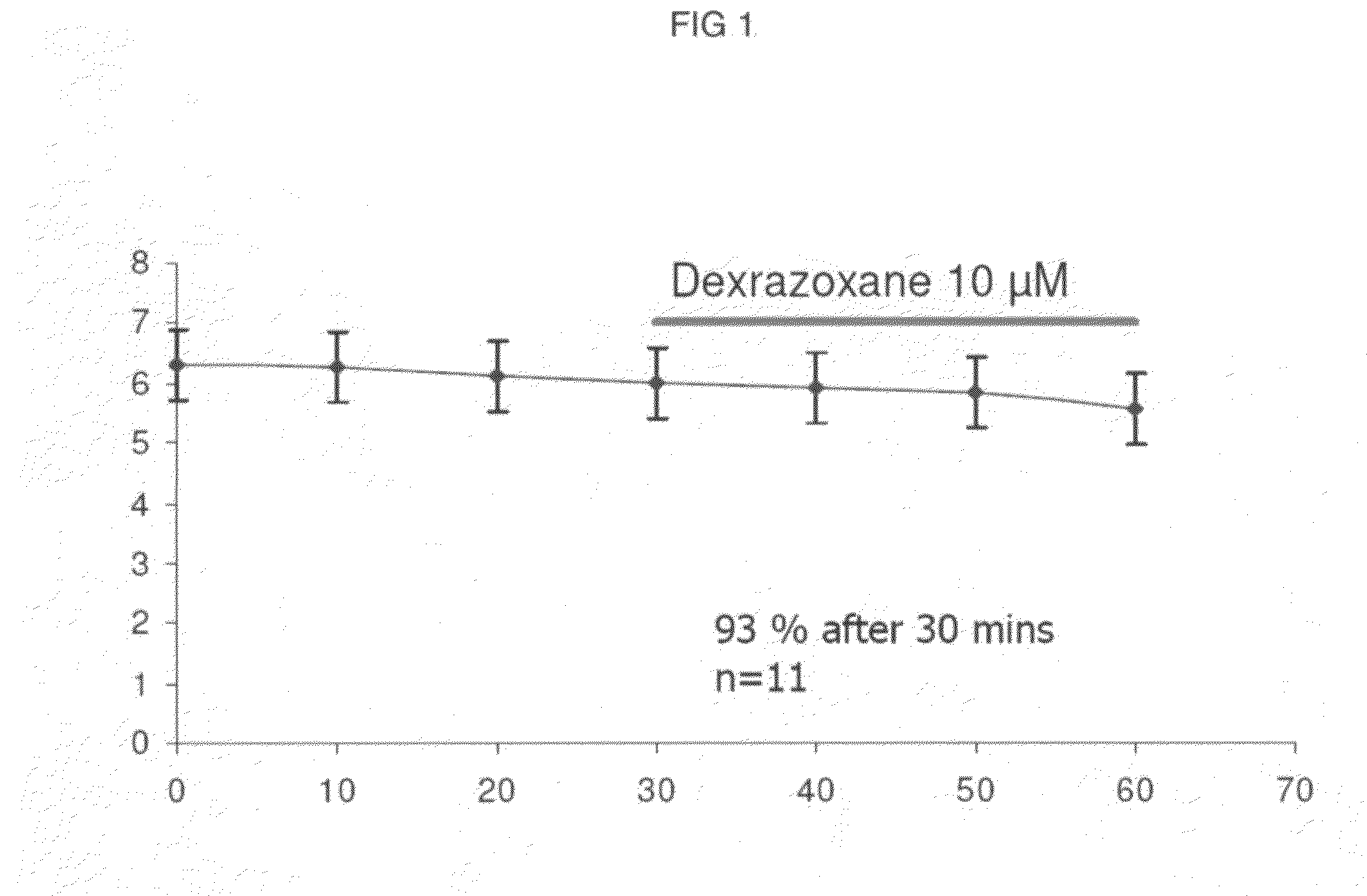
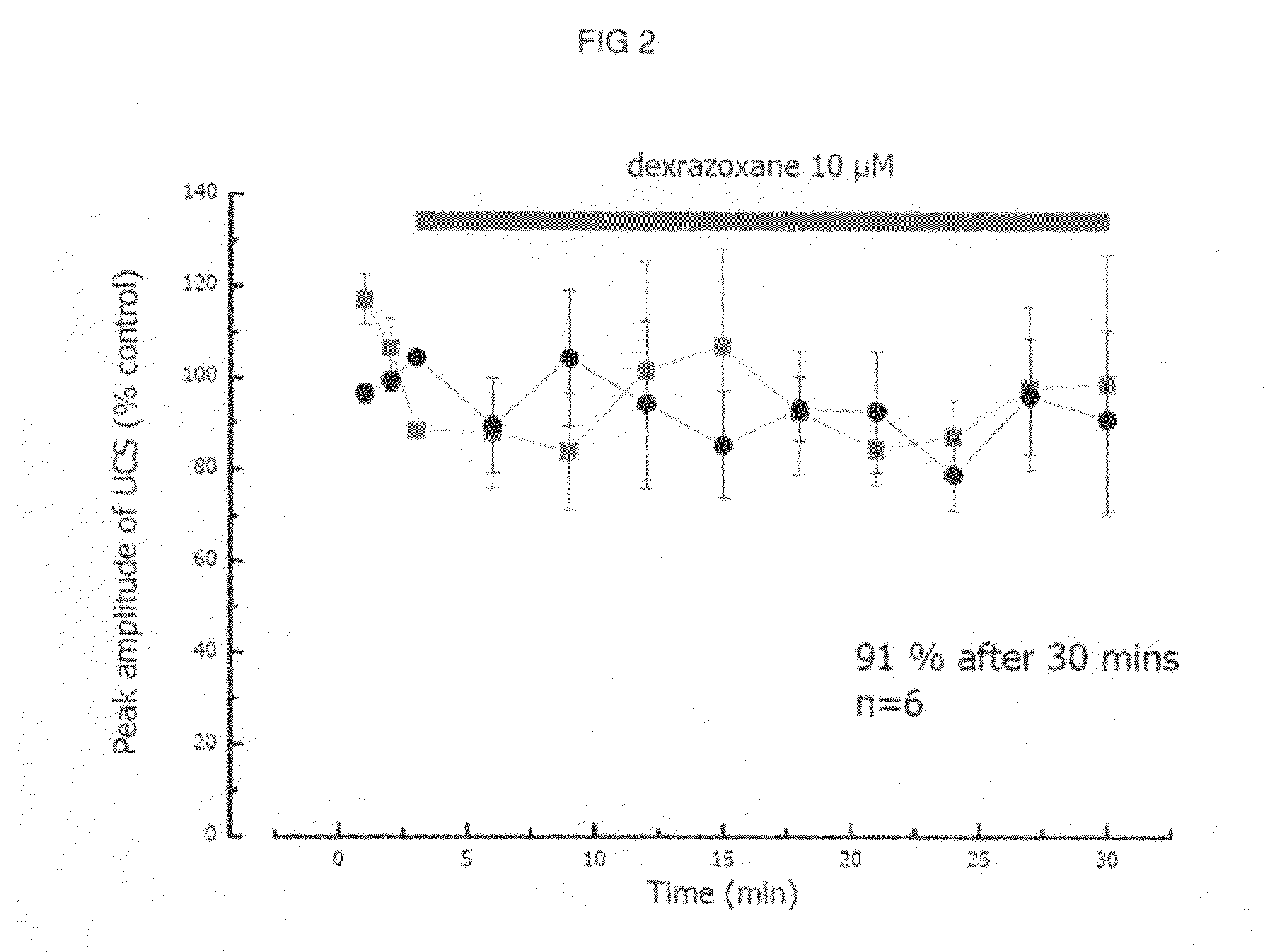
The present invention provides a method of treating conditions associated with iron and calcium overload comprising administering an effective amount of dexrazoxane or a non-dexrazoxane compound of formula (IA), (IB), or (IC) or a pharmaceutically acceptable salt, tautomer, or stereoisomer thereof.
Owner:APT PHARMA INC
Powder mixture composition of natural materials for controlling deficiencies in polypeptide alpha and beta hemoglobin chains
InactiveUS20140220163A1Reduce needReduces/eliminates side effectBiocideAnimal repellantsPowder mixtureBone marrow transplant
The embodiments herein provide a powdered composition of natural substances for controlling deficiencies in polypeptide alpha and beta hemoglobin chains and a diet plan for treating thalassemia patient. The composition comprises passargad powder, pars powder, purgative material, fumigation material, snuffing material, vinegar syrup and parsomash powder. The passargad powder is a stomach tonic and acts as anti-parasitic, anti-diarrheal, anti-dysentery, anti-bloating and anti-heartburn agents. The pars powder comprises sprouted grains for providing nutrition to cells. The purgative material is body purifier or detoxification agent and helps in motion and bowl movement. The vapor / fumigation material softens and reduces stickiness of sputum and removes sputum. The snuff helps in sneezing and removal of sputum. The vinegar syrup breaks down fat and removes toxins. The parsomash is an detoxification agent. The composition and dietary plan eliminates the need of bone marrow transplant, blood transfusion and defroxamin in thalassemia patients.
Owner:SOLEIMANI BABADI HAMZEH
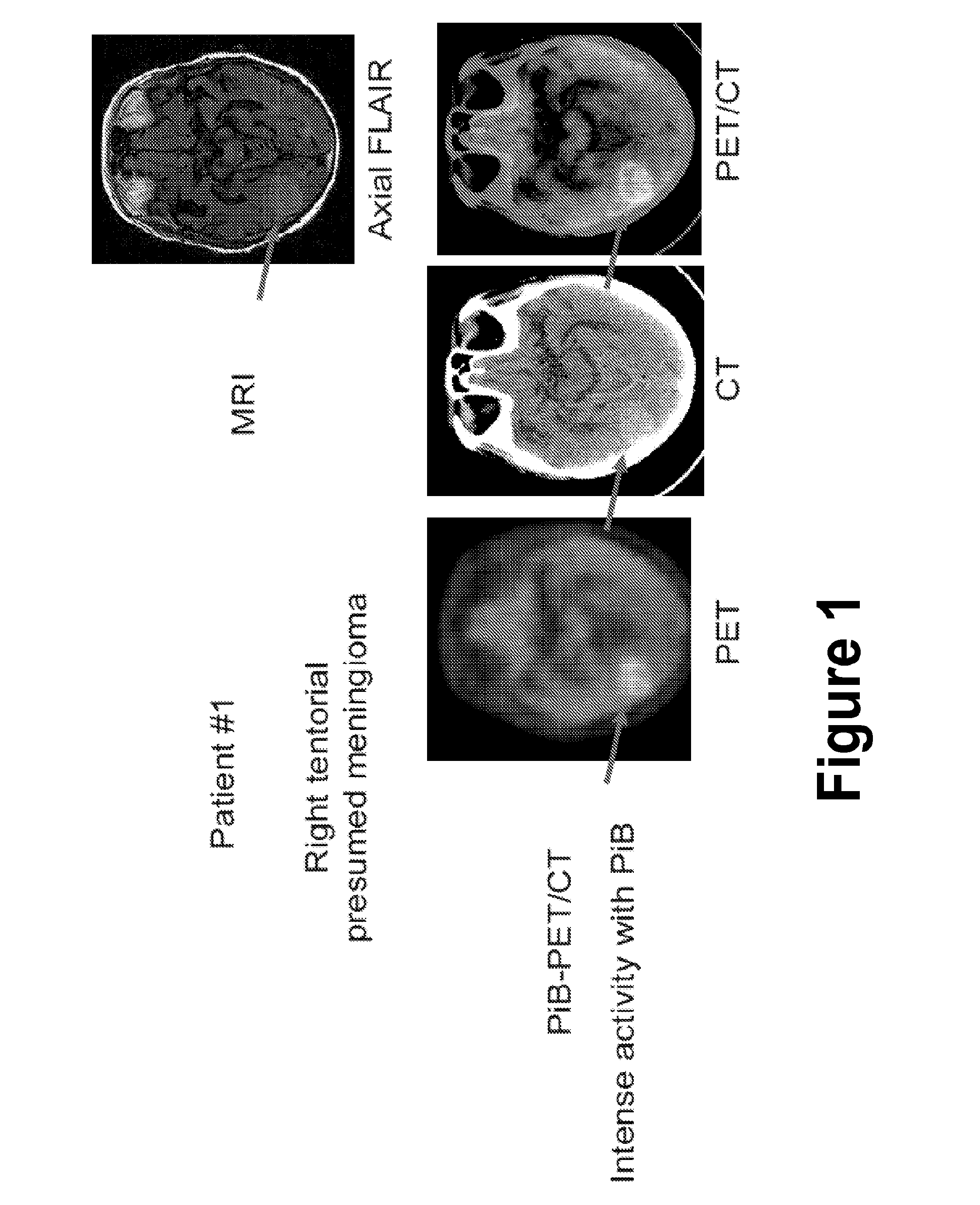
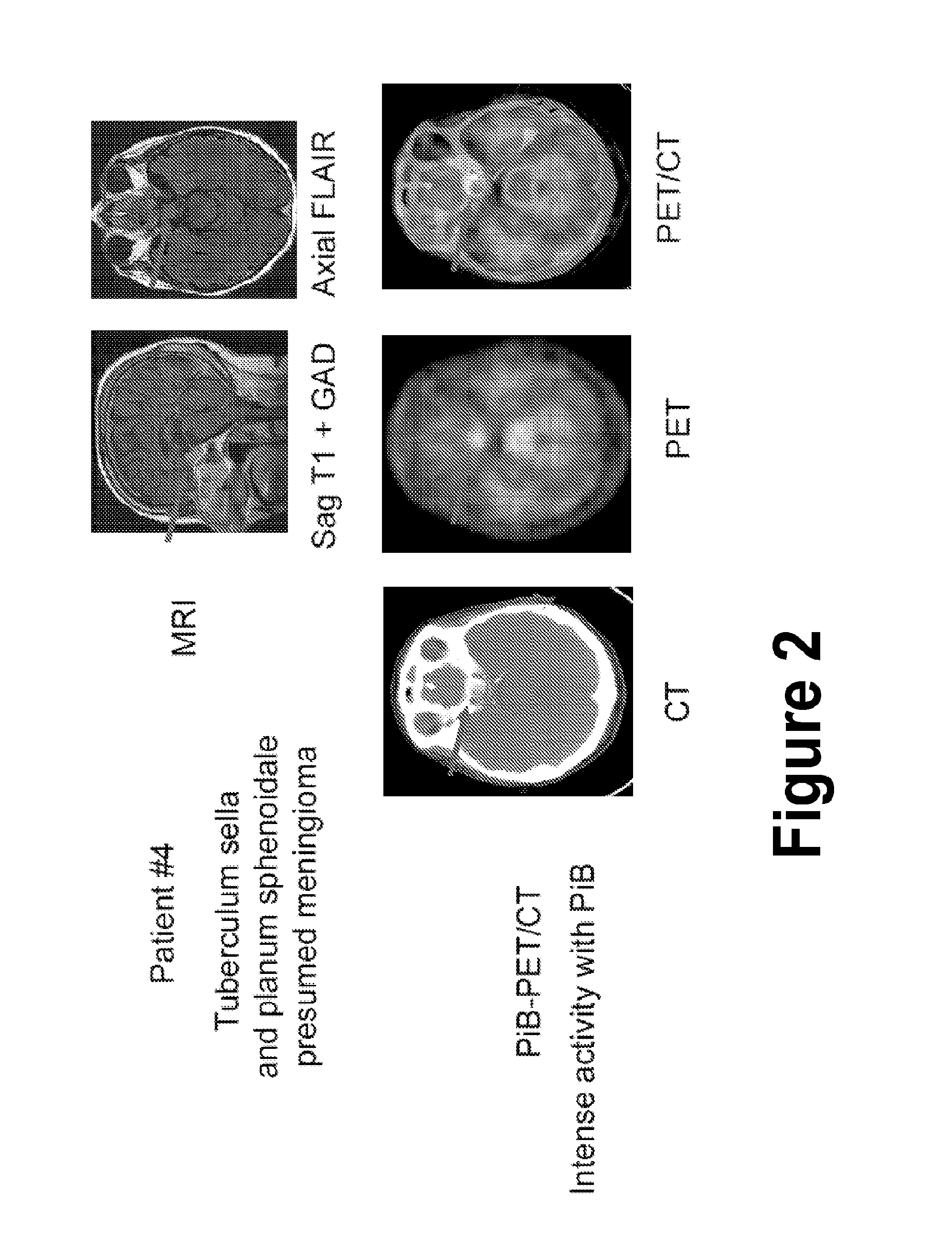
Treatment of meningiomas using phenylbenzothiazole, stilbene, biphenylalkyne, or pyridine derivatives
InactiveUS20150157744A1Side effectLess successfulOrganic active ingredientsOrganic chemistryPyridineCancer research
A method and composition for treating a meningioma in a subject are disclosed. The method includes the step of administering to the subject a therapeutically effective amount of a composition including a cytotoxic agent associated with a phenylbenzothiazole derivative or a stilbene derivative or a biphenylalkyne derivative that accumulates within meningiomas. In one version of the method, the phenylbenzothiazole derivative is a compound of formula (V).
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
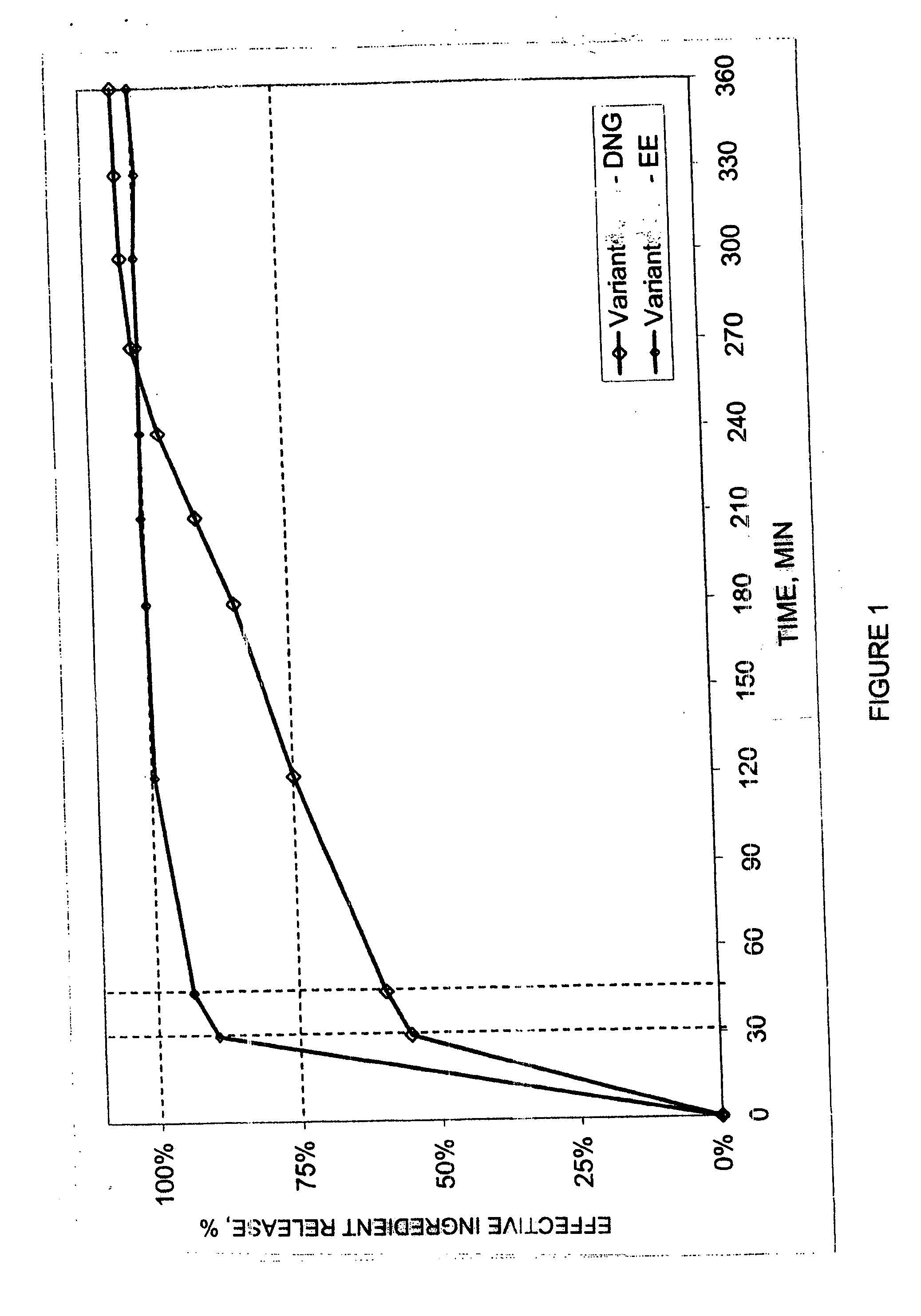
Pharmaceutical preparation for oral contraception
InactiveUS20060183725A1Effective contraceptive actionEasy cycle controlBiocideOrganic active ingredientsOral medicationAdditive ingredient
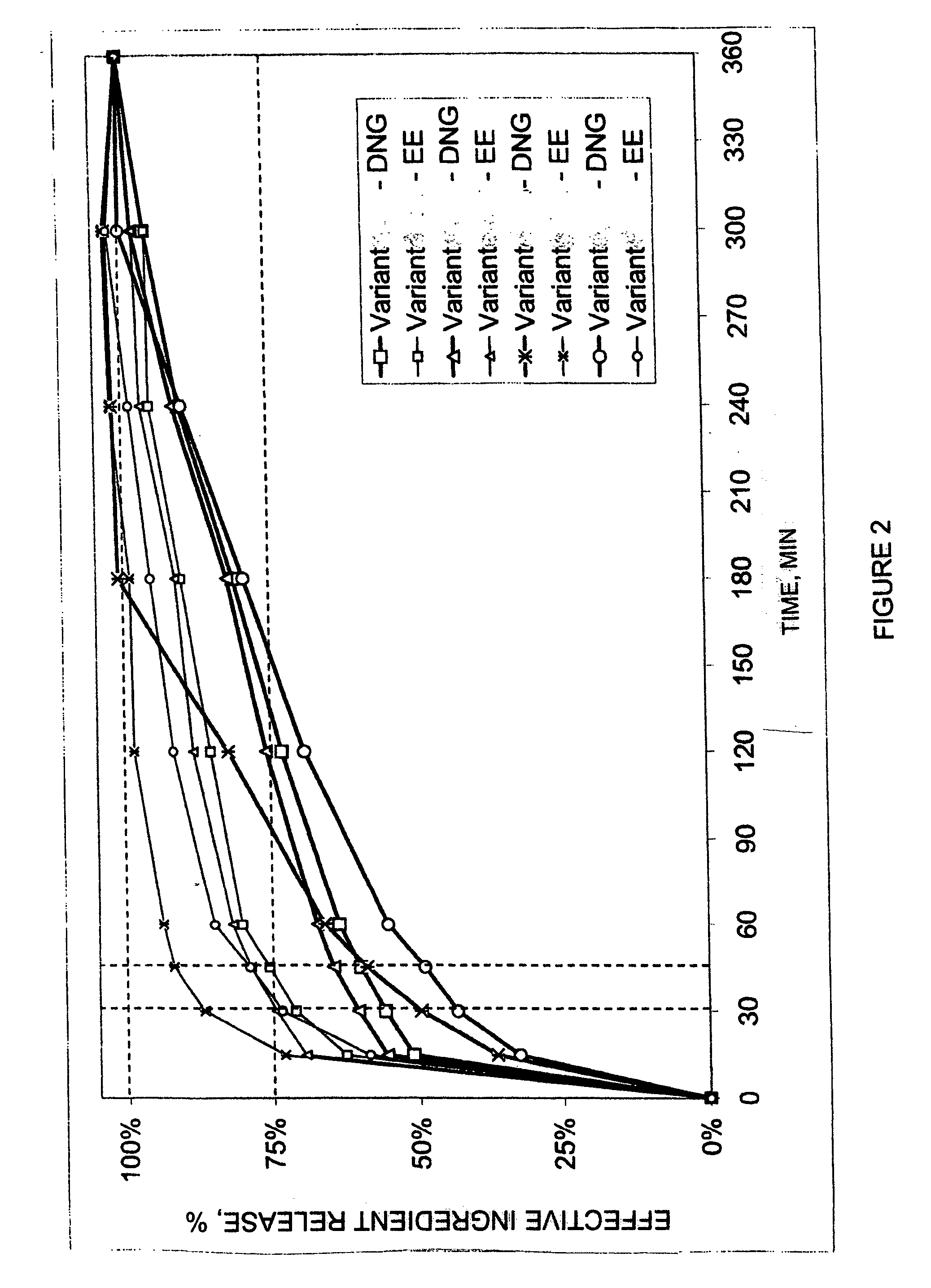
The pharmaceutical preparation for oral contraception has 28 daily dosage units, of which at least 21 daily dosage units each contain from 1.5 mg to 2 mg of dienogest and from 0.015 mg to 0.02 mg of ethinyl estradiol together in a pharmaceutically acceptable carrier. Seven or fewer daily dosage units contain no effective ingredient. Each daily dosage unit can be a film tablet for oral administration, which has a tablet core and film coating on the tablet core. At least 30% of the dienogest is released from the tablet core preferably in a delayed manner after more than 30 minutes, while at least 70% of the dienogest and ethinyl estradiol are released from the film coating preferably in 30 minutes, as determined by a standard dissolution test.
Owner:SCHERING AG
Therapeutic or Preventive Agent for Diabetes
ActiveUS20130303448A1Promote absorptionSide effectDipeptide ingredientsMetabolism disorderSecretionPeptide
A collagen peptide mixture containing three or more kinds selected from Glu-Hyp-Gly, Glu-Hyp, Leu-Hyp-Gly, Pro-Ala, Ser-Hyp, Ala-Hyp-Gly, chemically-modified substances thereof and pharmaceutically acceptable salts thereof, and at least one peptide selected from the group consisting of Glu-Hyp-Gly, Glu-Hyp, Leu-Hyp-Gly, Pro-Ala, Ser-Hyp, Ala-Hyp-Gly, Pro-Hyp-Gly, Leu-Hyp, Ile-Hyp, Ser-Hyp-Gly, Gly-Pro-Hyp, (Pro-Hyp-Gly)5, Pro-Hyp, Hyp-Gly, Pro-Gly, Pro-Pro and Ala-Hyp or a chemically-modified substance thereof or a pharmaceutically acceptable salt thereof have DPPTV inhibitory activity and / or GLP-1 secretion accelerating activity, and hence are effective as a therapeutic or preventive agent or the like for diabetes.
Owner:NITTA GELATIN INC
Catalytic System for Polymerisation of Lower Alpha Alkene
InactiveUS20070260099A1Without side effectNon toxicOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationCarboxylic acidDicarboxylic acid
Catalytic system for polymerization of lower alpha alkene including a selectivity control agent which consists of naturally derived pure isomers of tartrates such as esters of (2-R,3-R)-dihydroxy-butane-1,4dicarboxylic acid or (2-S,3-S)-dihydroxybutane-1,4-dicarboxylic acid. The molar ratio of the optically pure isomers of the tartrates to titanium being 0.0375 to 1.5.
Owner:RELIANCE INDUSTRIES LIMITED
Perfusion device and method for operating same
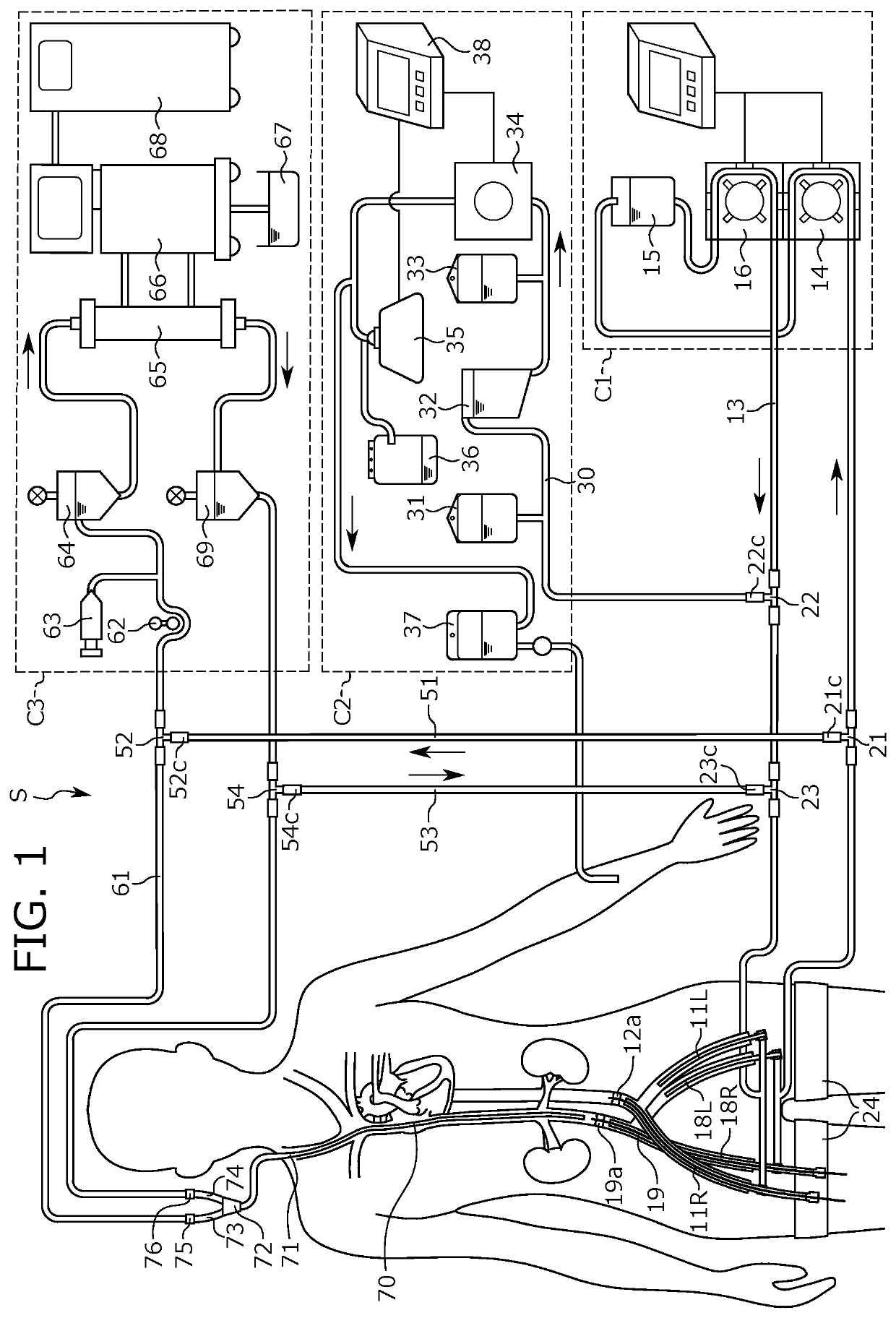
ActiveUS20200197588A1Enhance effectSide effectOther blood circulation devicesDialysis systemsBiomedical engineeringRat heart
A negative-balanced isolated pelvic perfusion method, in which a drug is administered into the closed pelvis while keeping the volume of suction from the vein larger than that of injection into the artery, does not require allogeneic blood transfusion. A perfusion device is for recovering a liquid containing a drug and / or blood from a tube connecting to the inferior vena cava and for injecting the liquid obtained into a tube connecting to the artery, provided with a unit for closing the inside of the pelvis by including a unit for blocking the artery from the heart to the pelvis, a unit for blocking the inferior vena cava from the pelvis to the heart, and a unit for blocking a blood flow from the pelvis to the lower limbs. The perfusion device is provided with a pelvic perfusion unit equipped with a reservoir, an autotransfusion unit, and a dialysis unit.
Owner:KOSEI ADVANCE
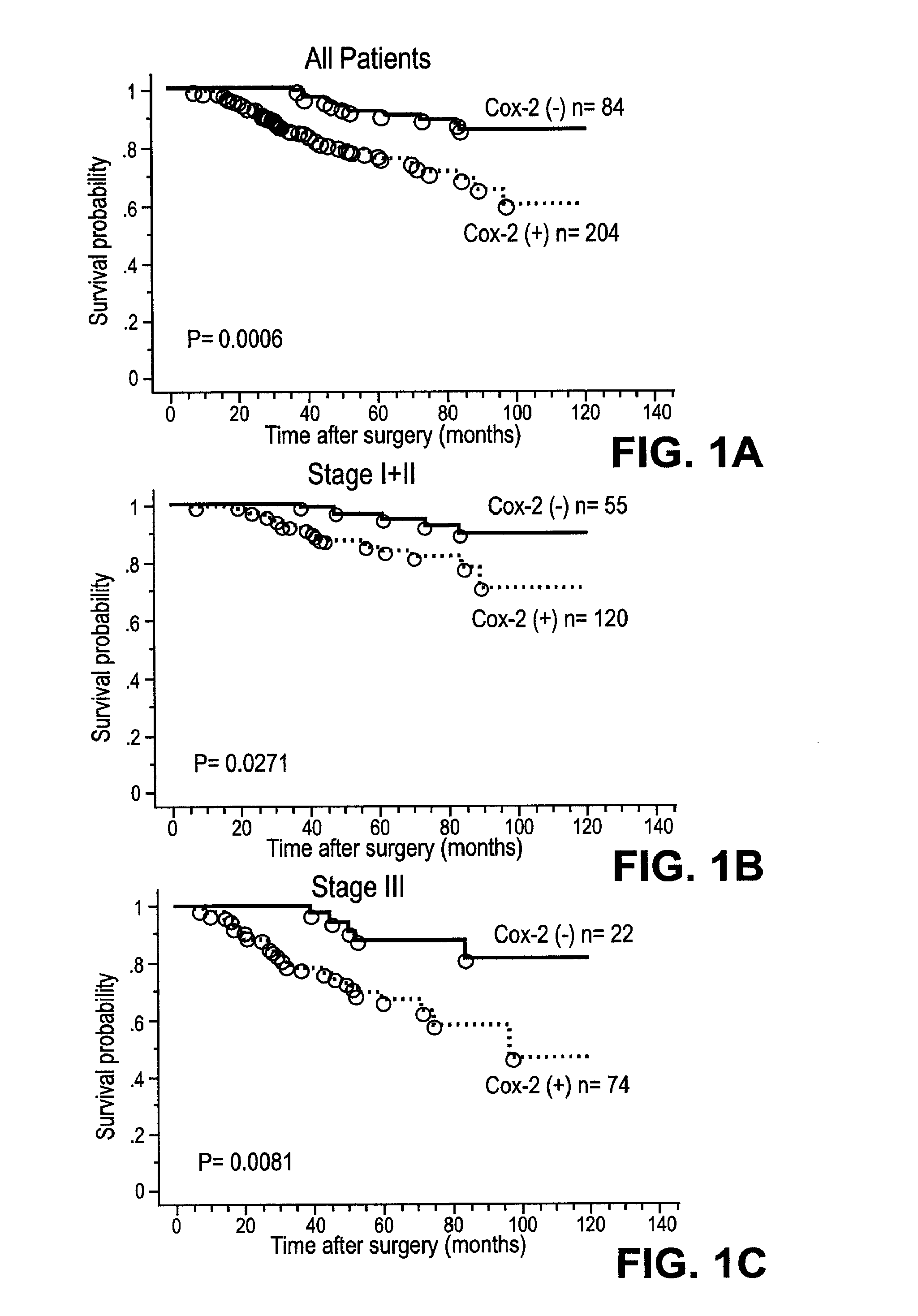
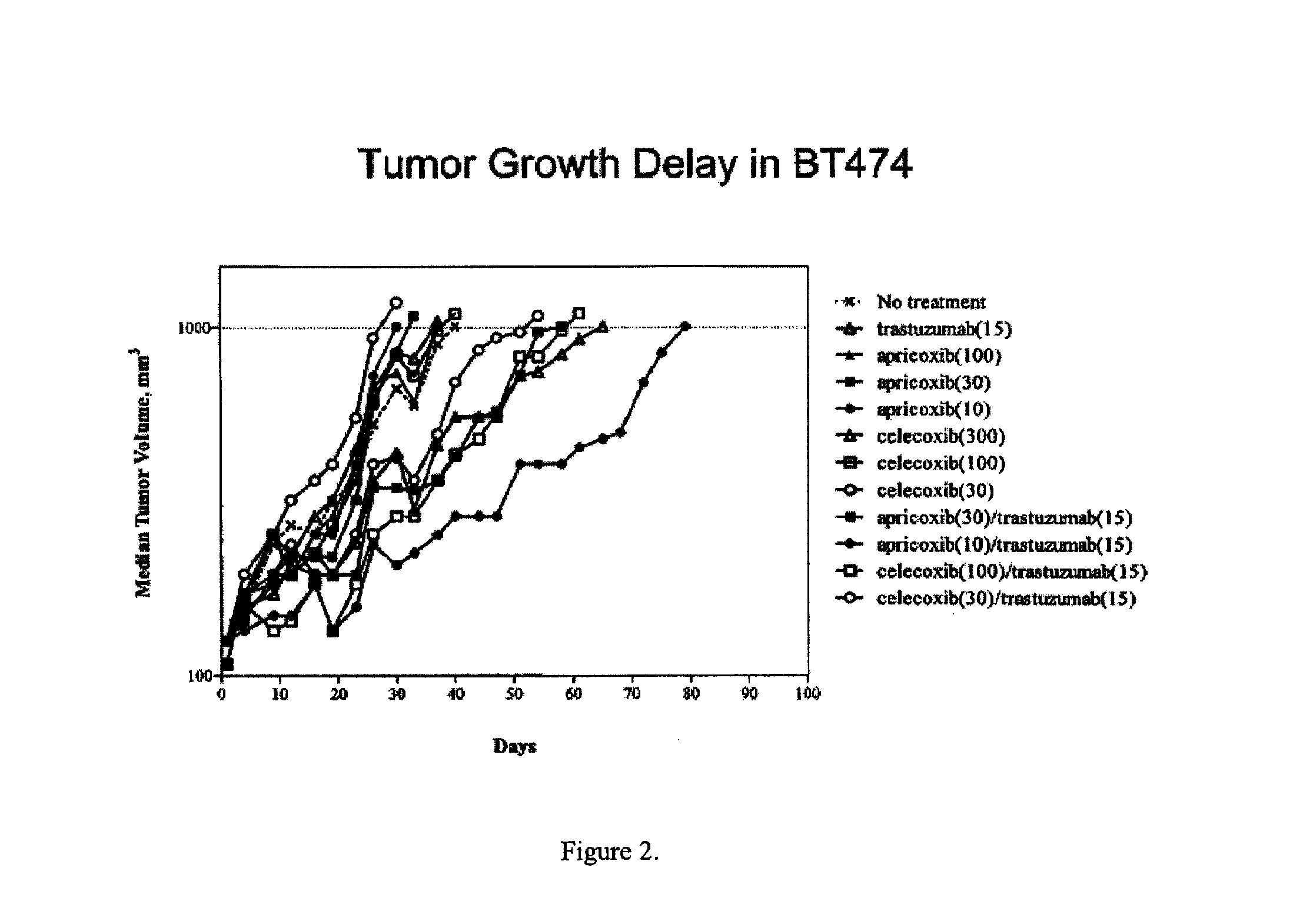
Therapies for treating cancer using combinations of cox-2 inhibitors and Anti-her2(ERBB2) antibodies or combinations of cox-2 inhibitors and her2(ERBB2) receptor tyrosine kinase inhibitors
InactiveUS20120052061A1Great effectSide effectBiocideMammal material medical ingredientsWilms' tumorAntibody
Described herein are compositions and methods for using these compositions in the treatment of cancer, tumors, and tumor-related disorders in a subject.
Owner:TRAGARA PHARMA INC
Application of radiofrequency catheter ablation system to treatment of essential hypertension
ActiveUS20180125572A1Blood pressure level be reduceReduce activitySurgical needlesCatheterNervous systemDisease
An application of a radiofrequency catheter ablation system to treatment of essential hypertension. The treatment process is under ultrasonic guidance, an electrode needle is made to penetrate into target tissue of a patient, electrification is conducted to start ablation, the needle is withdrawn or made to penetrate into the other side of the target tissue for target ablation after reaching ablation temperature and duration time, and overall ablation treatment is completed. The system can treat a secondary center for regulating the activity of a whole-body sympathetic system to reduce its activity, thus, the blood pressure level of a patient can be reduced, and fewer kinds and smaller dosage of antihypertensive drugs taken or ceased altogether. By treating the secondary center, the activity of the whole-body sympathetic system, insulin resistance and whole-body fibrosis is reduced, and other diseases characterized in activity abnormity of the sympathetic system might be treated too.
Owner:NANJING GUANGCI MEDICAL TECH
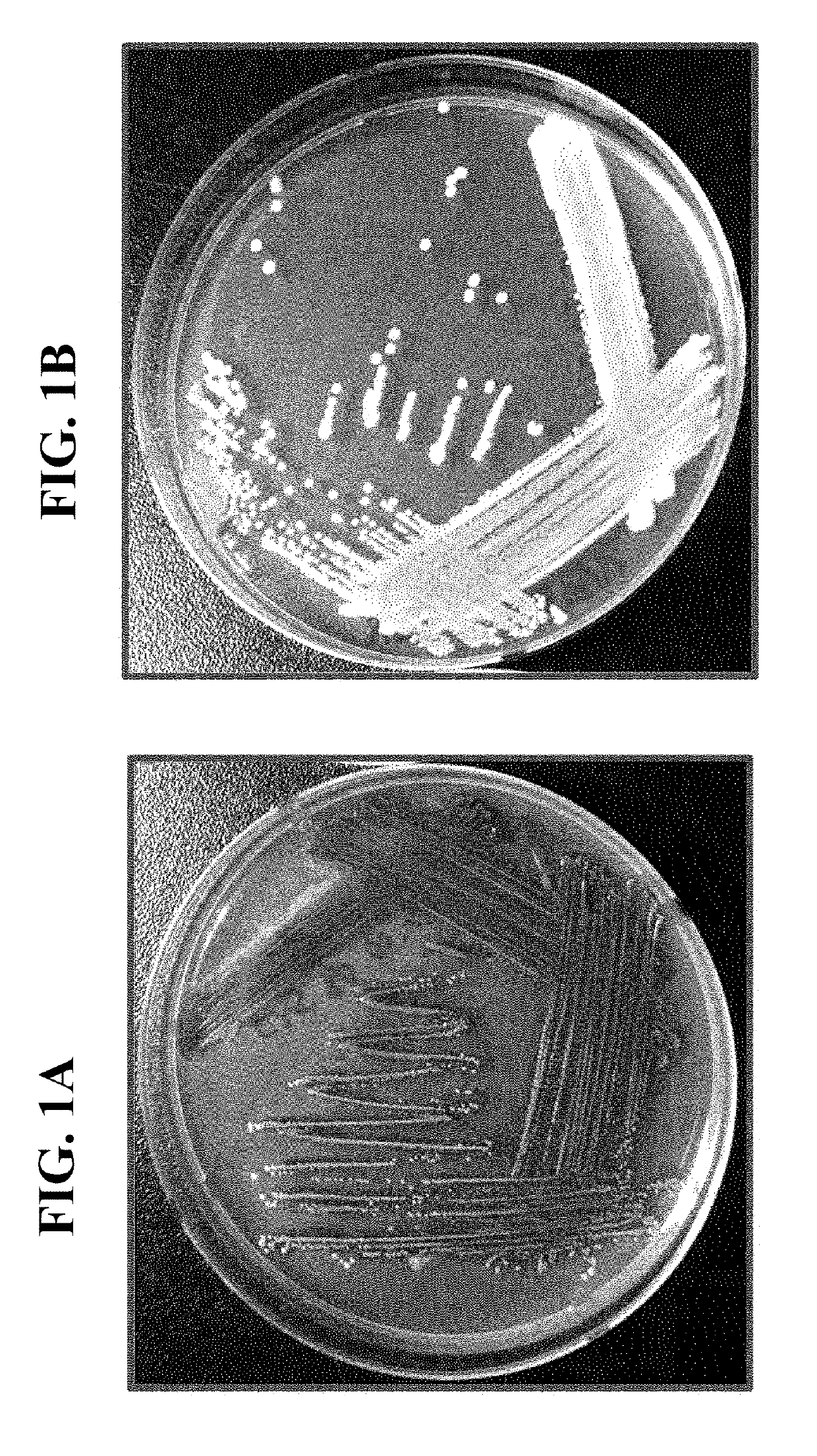
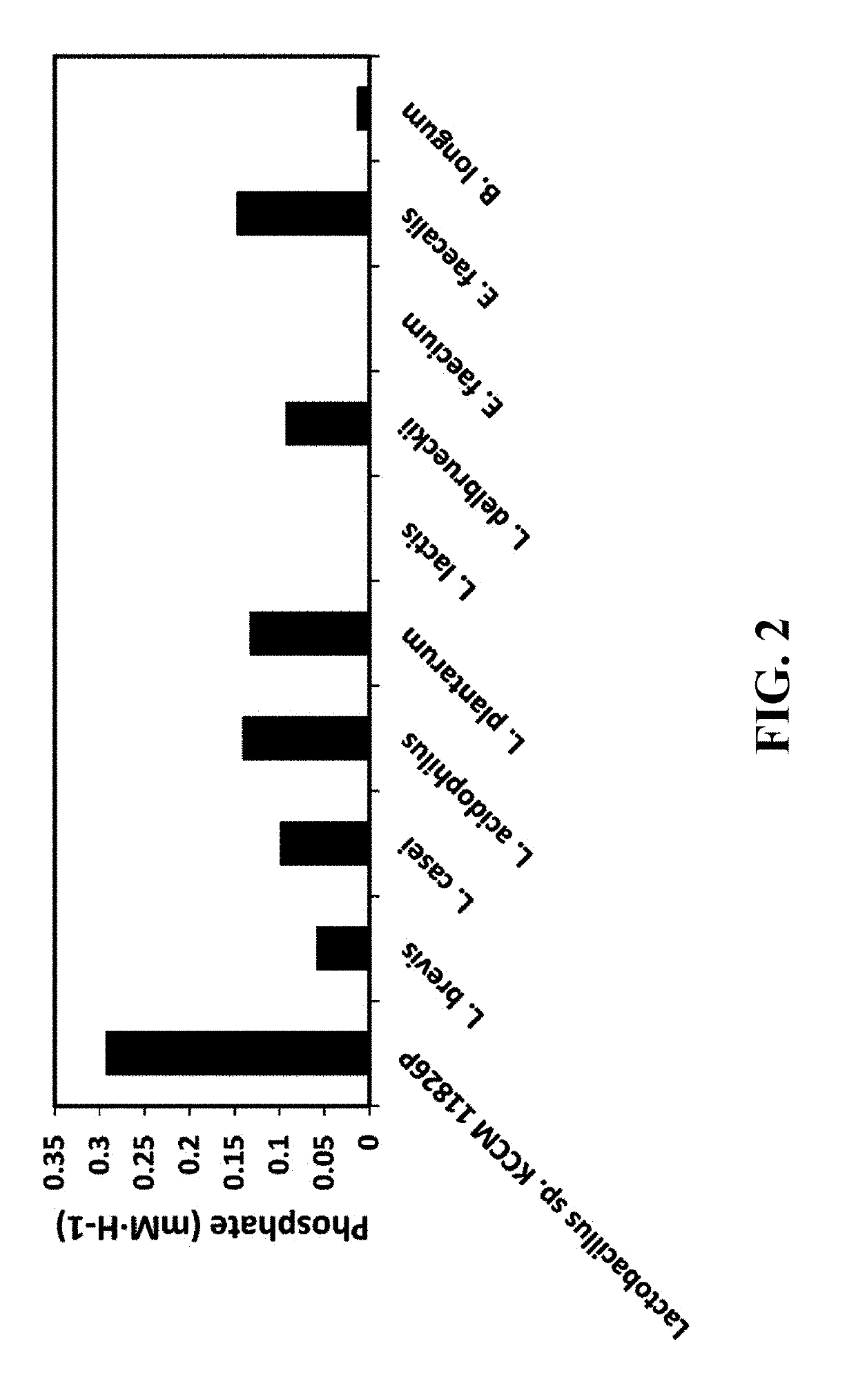
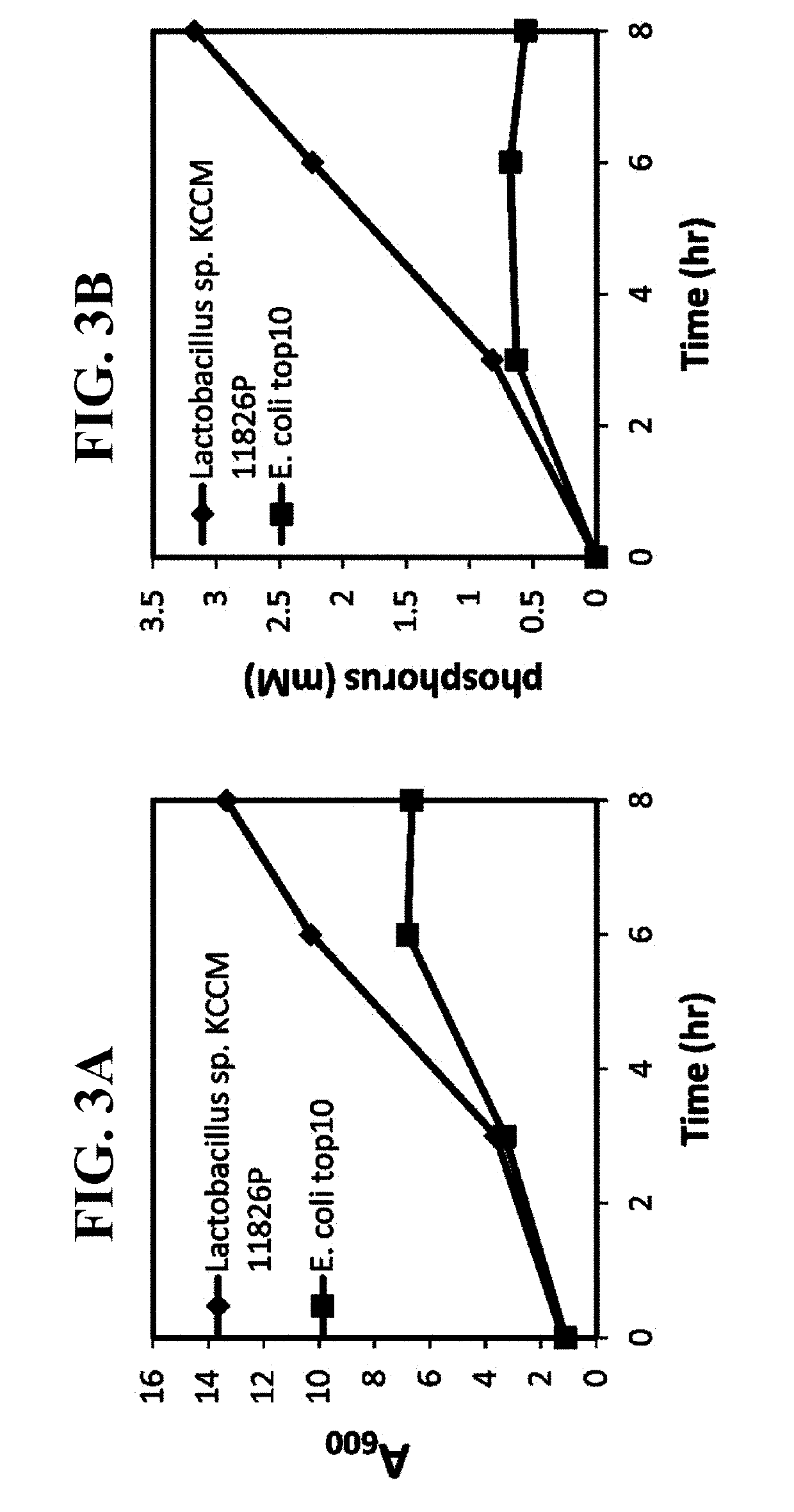
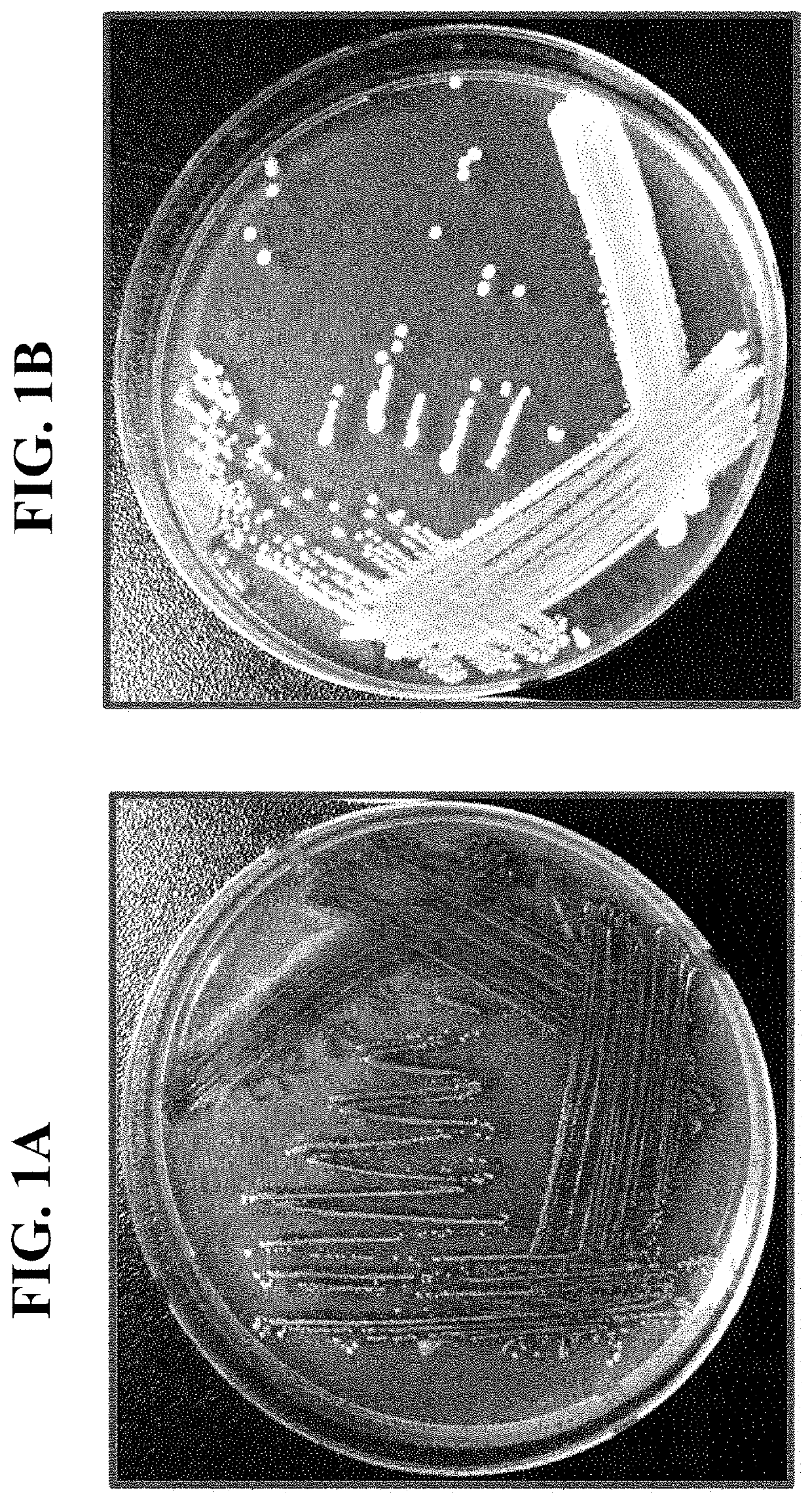
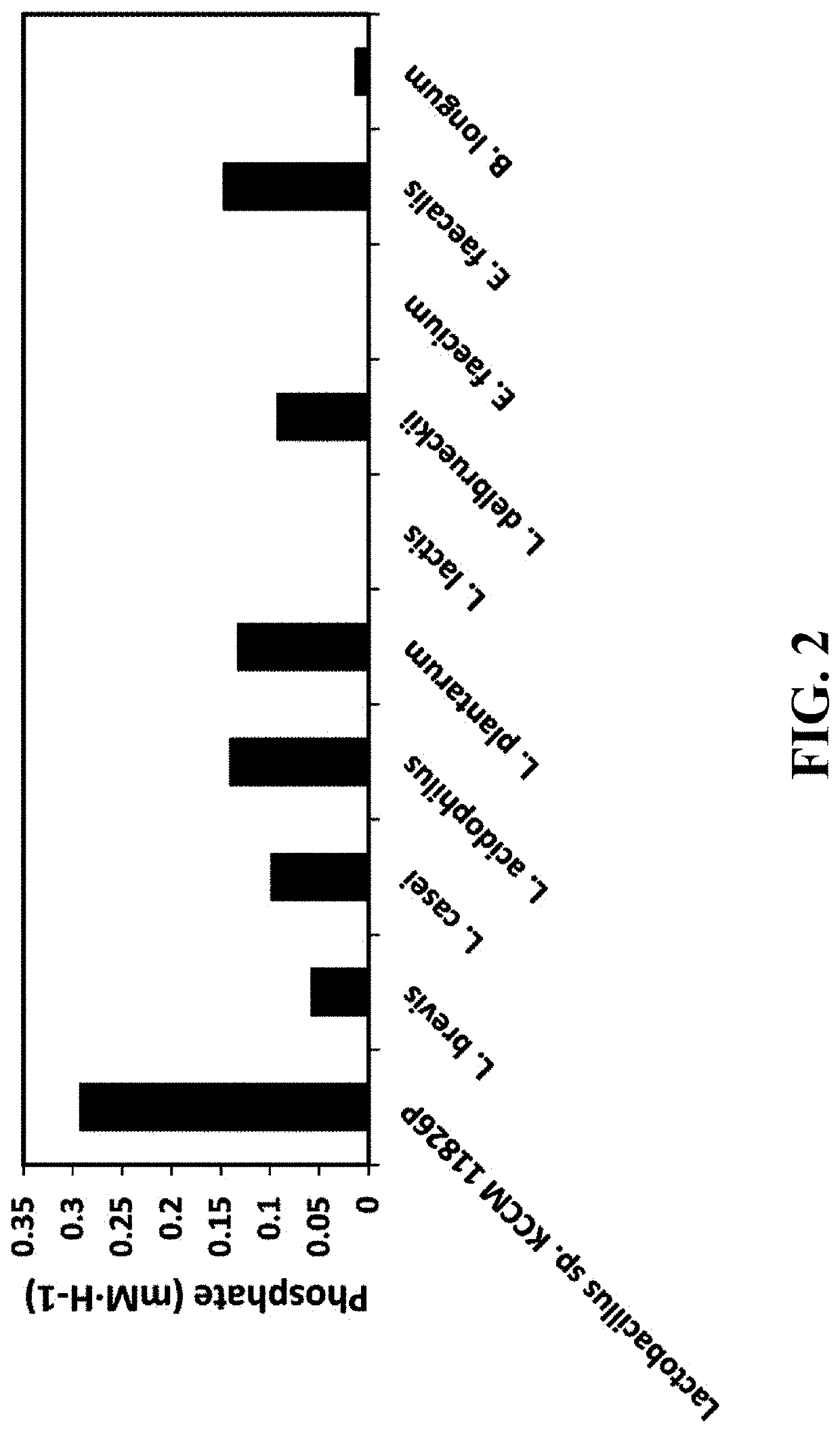
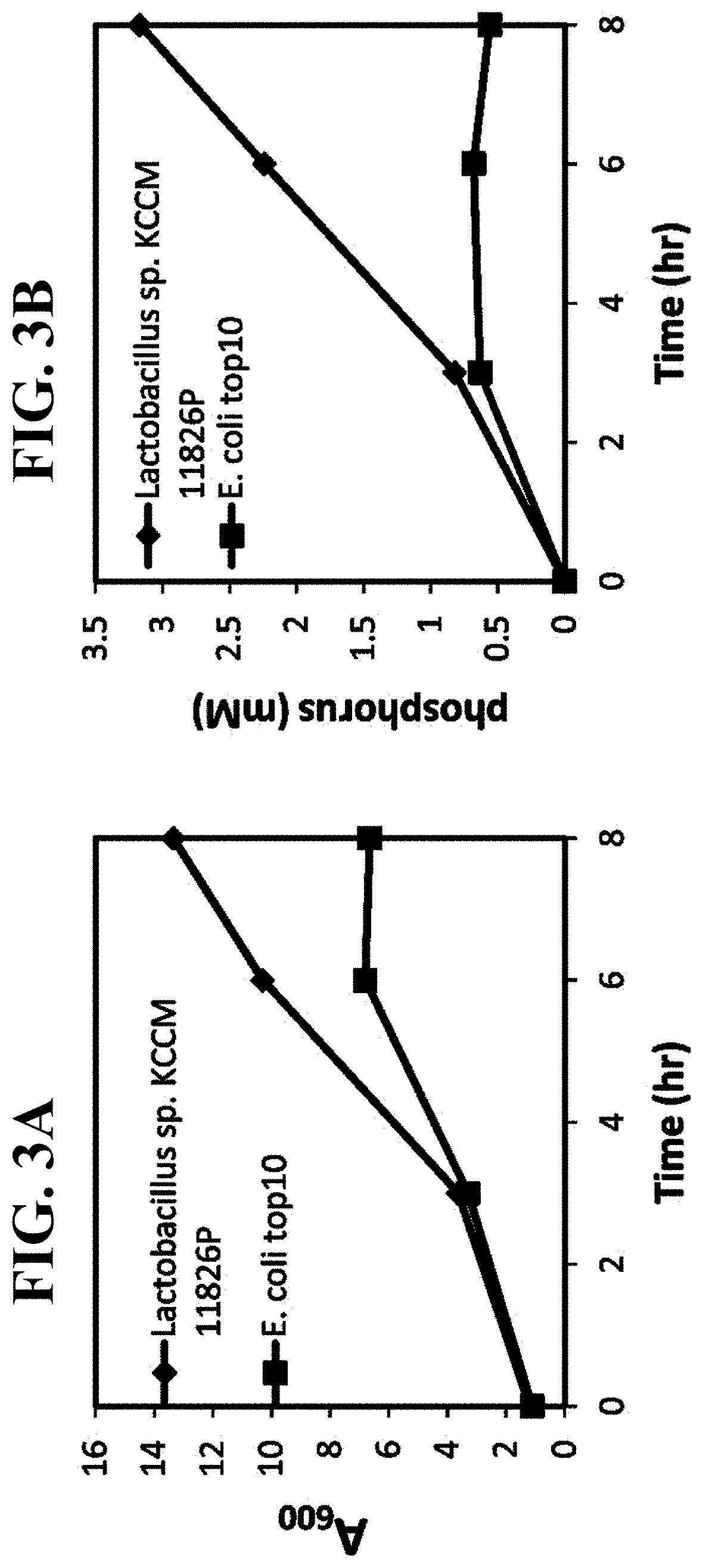
Pharmaceutical composition and healthy food composition with lactobacillus sp. kccm 11826p for preventing or treating hyperphosphatemia in chronic kidney disease
ActiveUS20190240270A1Improve abilitiesEffective controlMicroorganismsMicroorganism based processesNephropathyLactobacillus sp
Provided are a functional food or pharmaceutical composition or a method for preventing, alleviating or treating hyperphosphatemia and chronic kidney disease and treating chronic kidney disease using the composition comprising Lactobacillus sp. KCCM 11826P having excellent phosphorus-absorbing ability.
Owner:KOREA FOOD RES INST +1
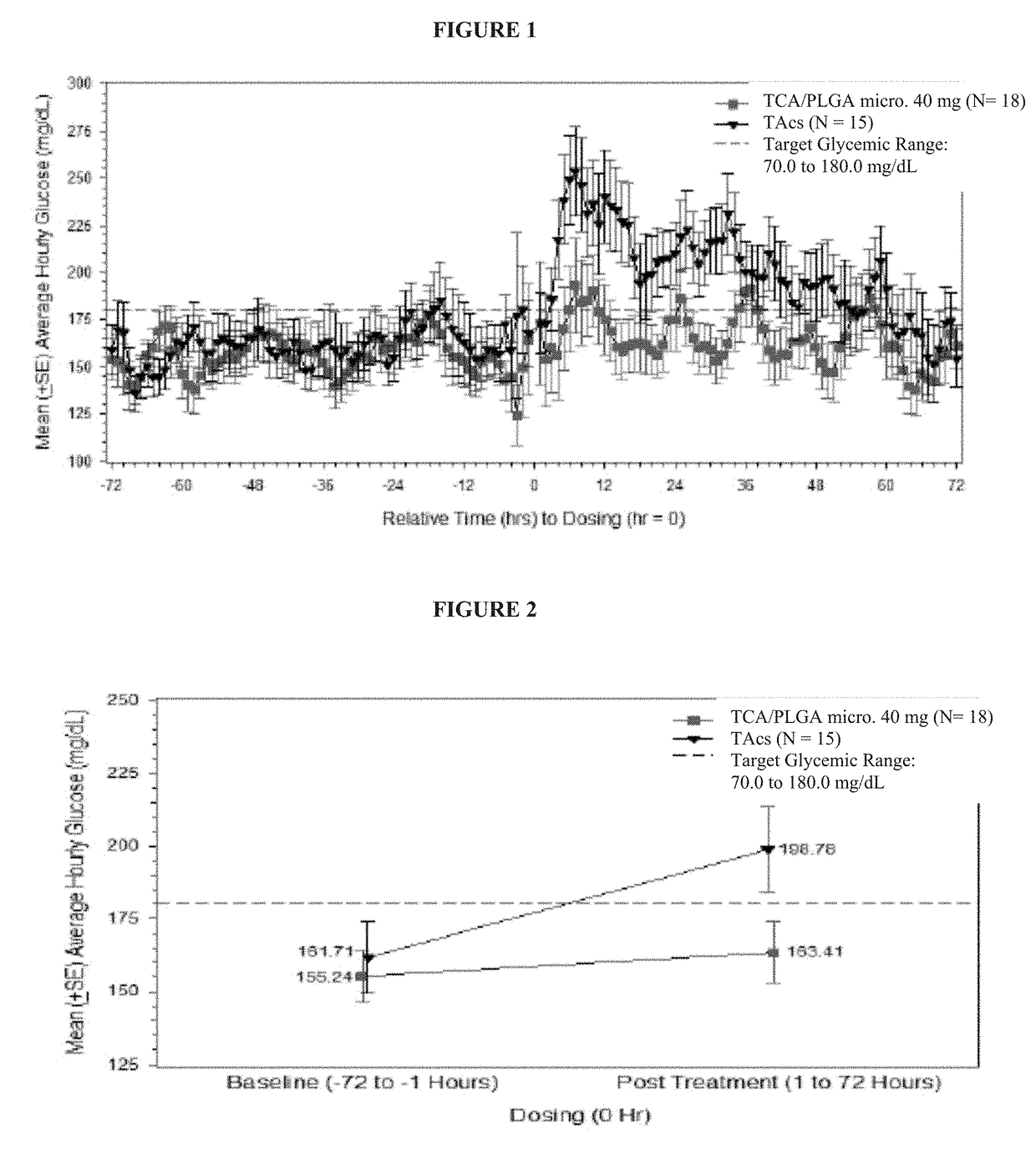
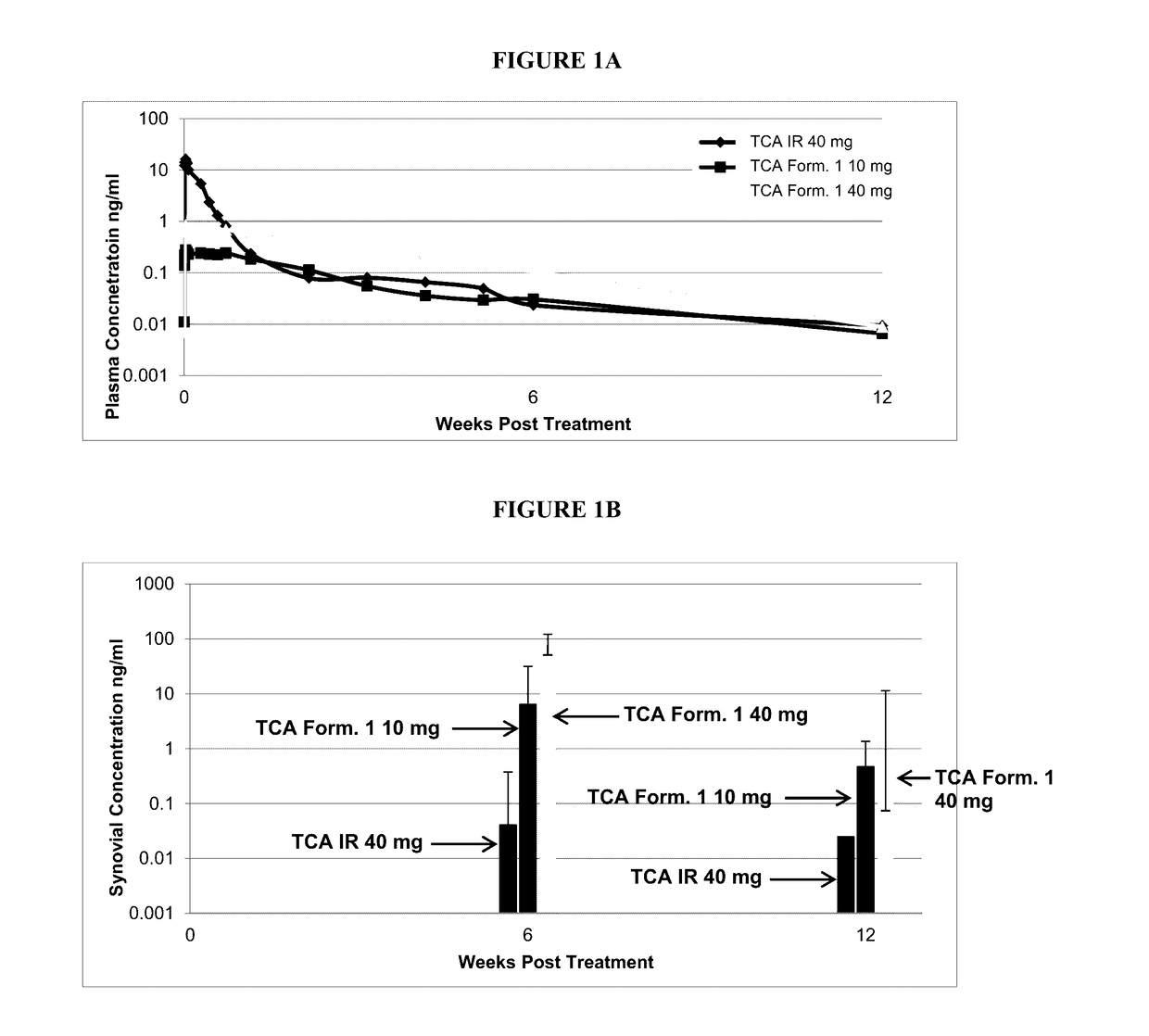
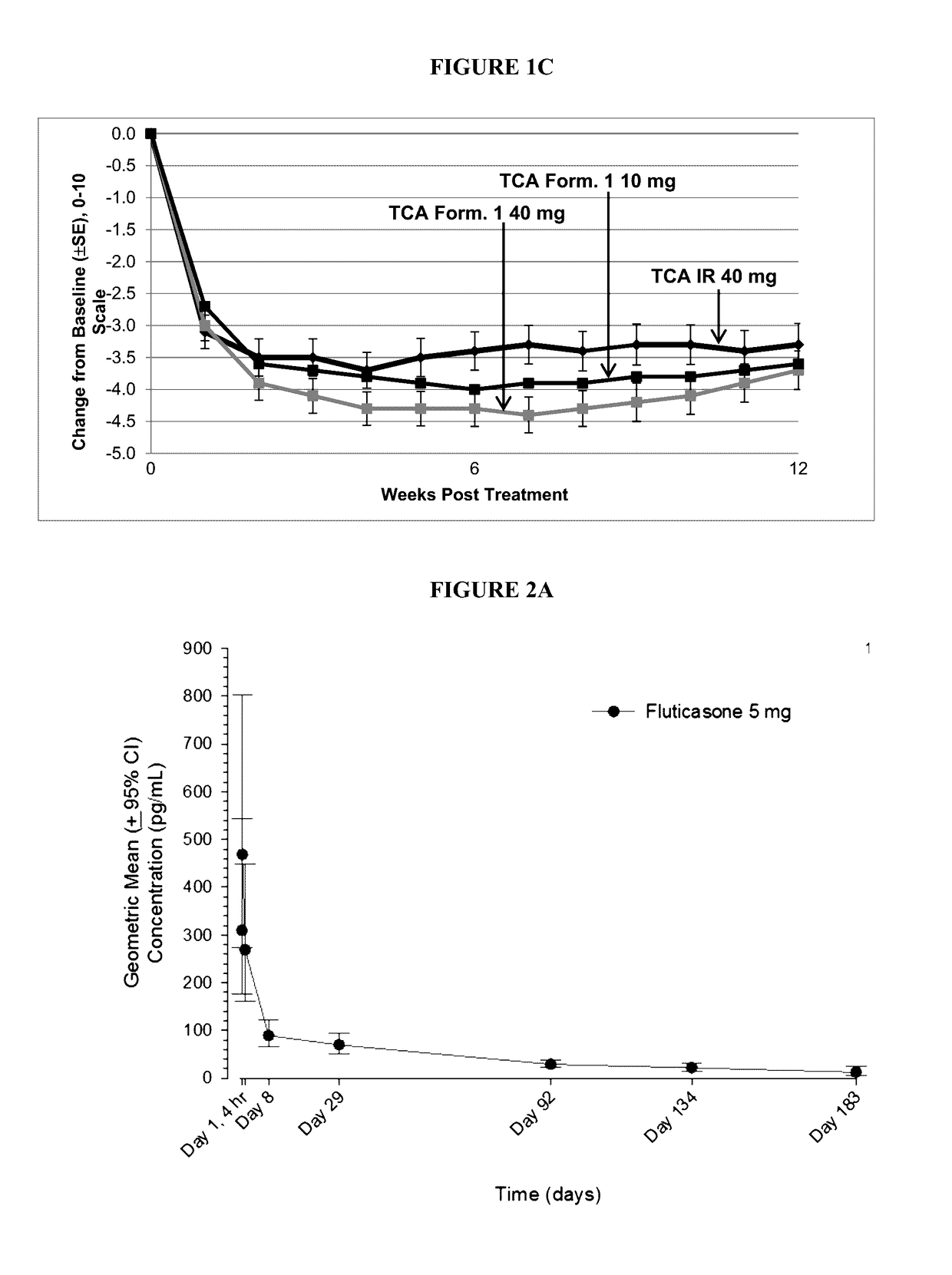
Corticosteroid formulations and methods for the treatment of joint pain in patients with diabetes
InactiveUS20170258815A1Reduce exposureProlong synovial residenceOrganic active ingredientsGranular deliveryCorticosteroid preparationImmediate release
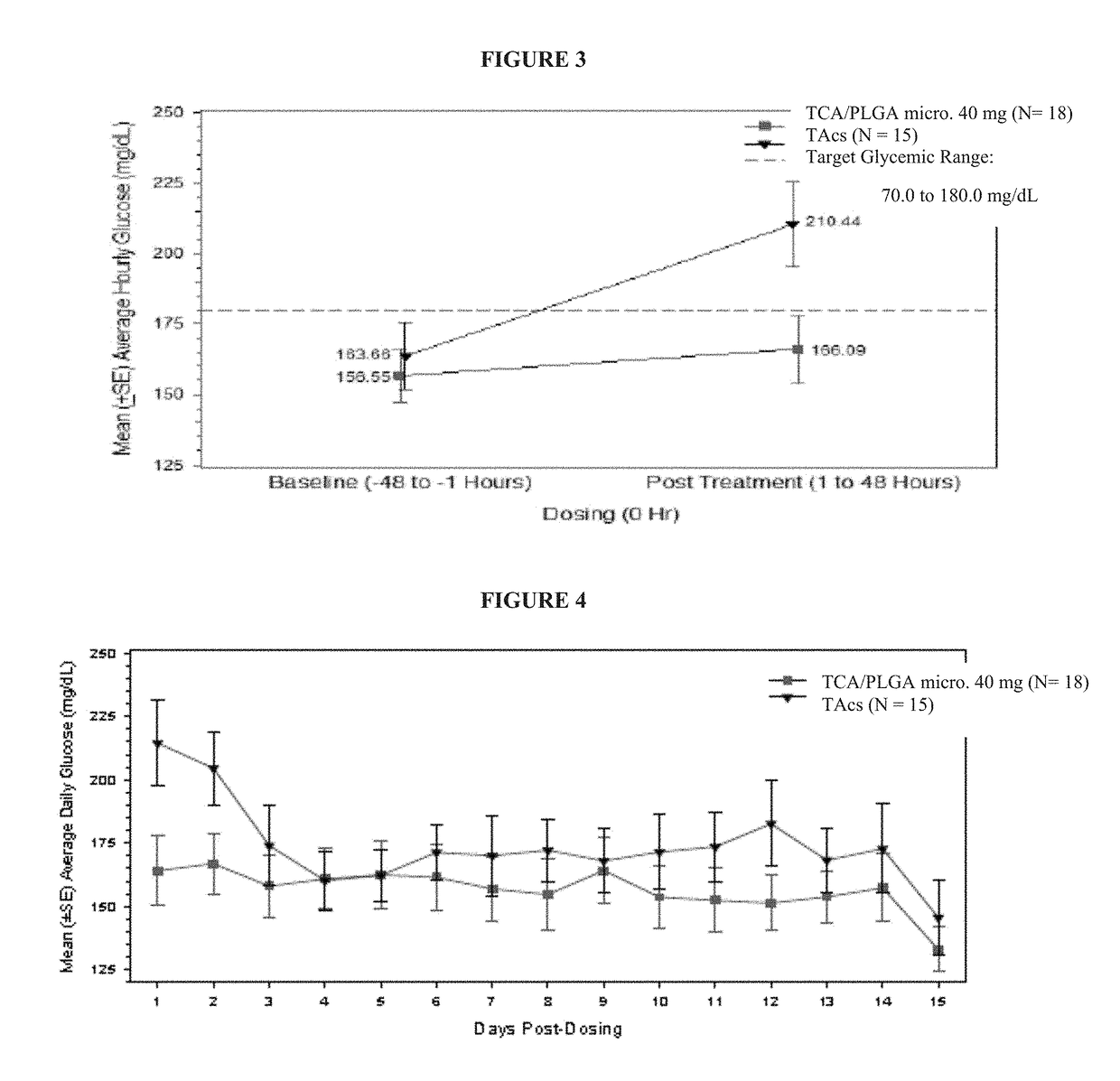
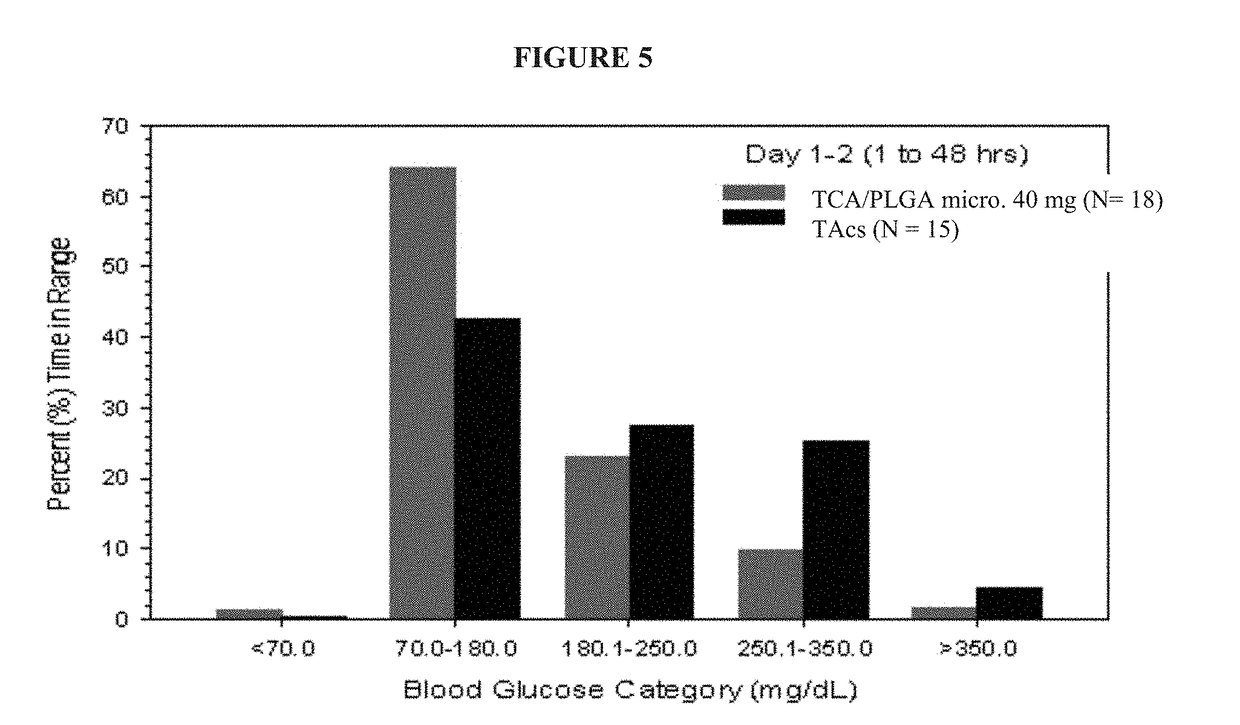
This invention relates to the use of corticosteroids in patients with diabetes, including patients with type 2 diabetes, to treat pain, including pain caused by inflammatory diseases such as osteoarthritis or rheumatoid arthritis without increasing or otherwise significantly impacting blood glucose concentrations in diabetic patients, and to slow, arrest or reverse structural damage to tissues caused by an inflammatory disease, for example damage to articular and / or periarticular tissues caused by osteoarthritis or rheumatoid arthritis without increasing or otherwise impacting blood glucose concentrations. More specifically, a formulation of triamcinolone acetonide (TCA) is administered locally to diabetes patients, including type 2 diabetes patients, as a sustained release dosage form (with or without an immediate release component) that results in efficacy levels accompanied by clinically insignificant or no measurable effect on blood glucose levels.
Owner:FLEXION THERAPEUTICS
Purification method
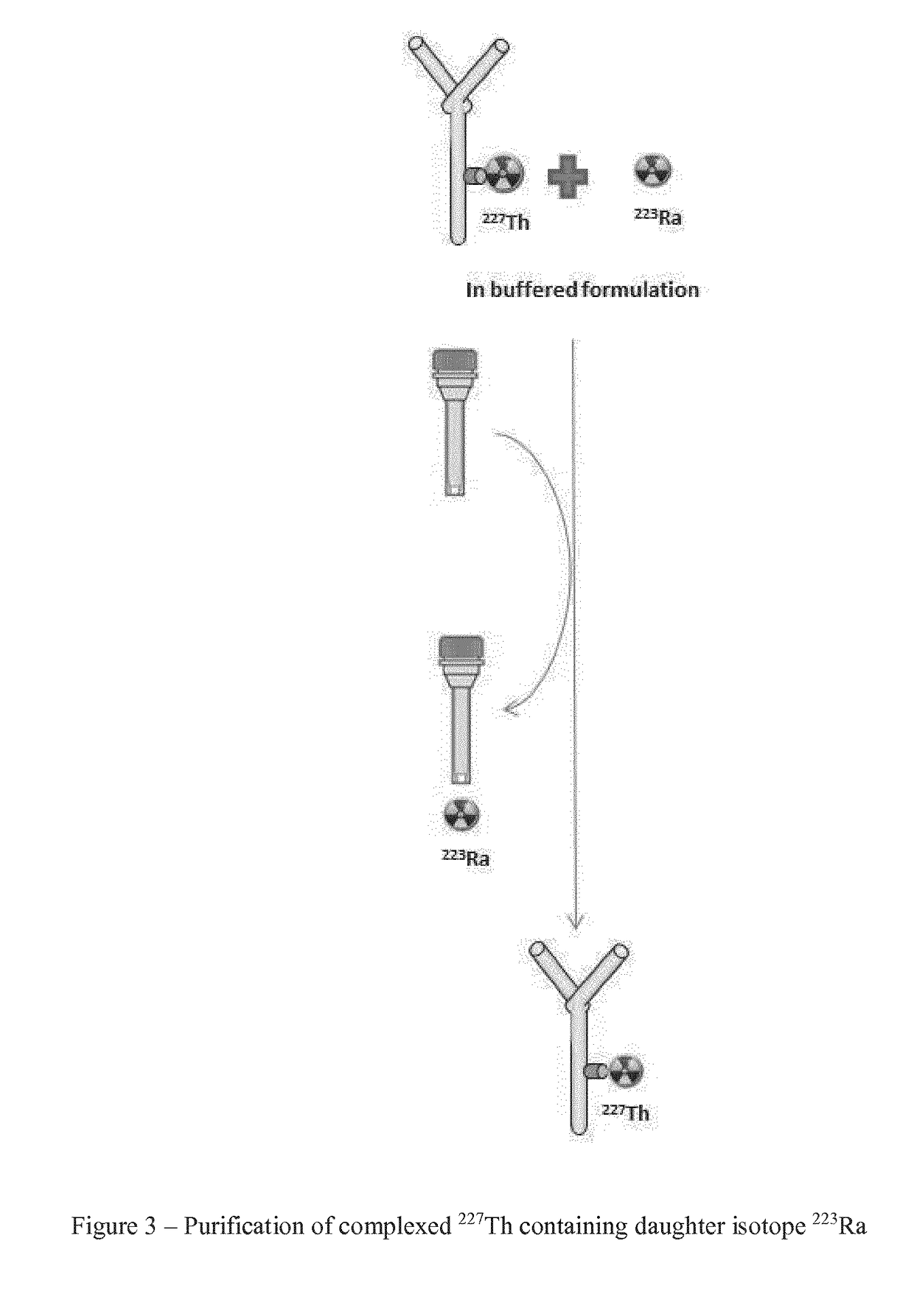
InactiveUS20190002363A1High radiochemical purityReduce delayCation exchanger materialsOrganic anion exchangersIonChemistry
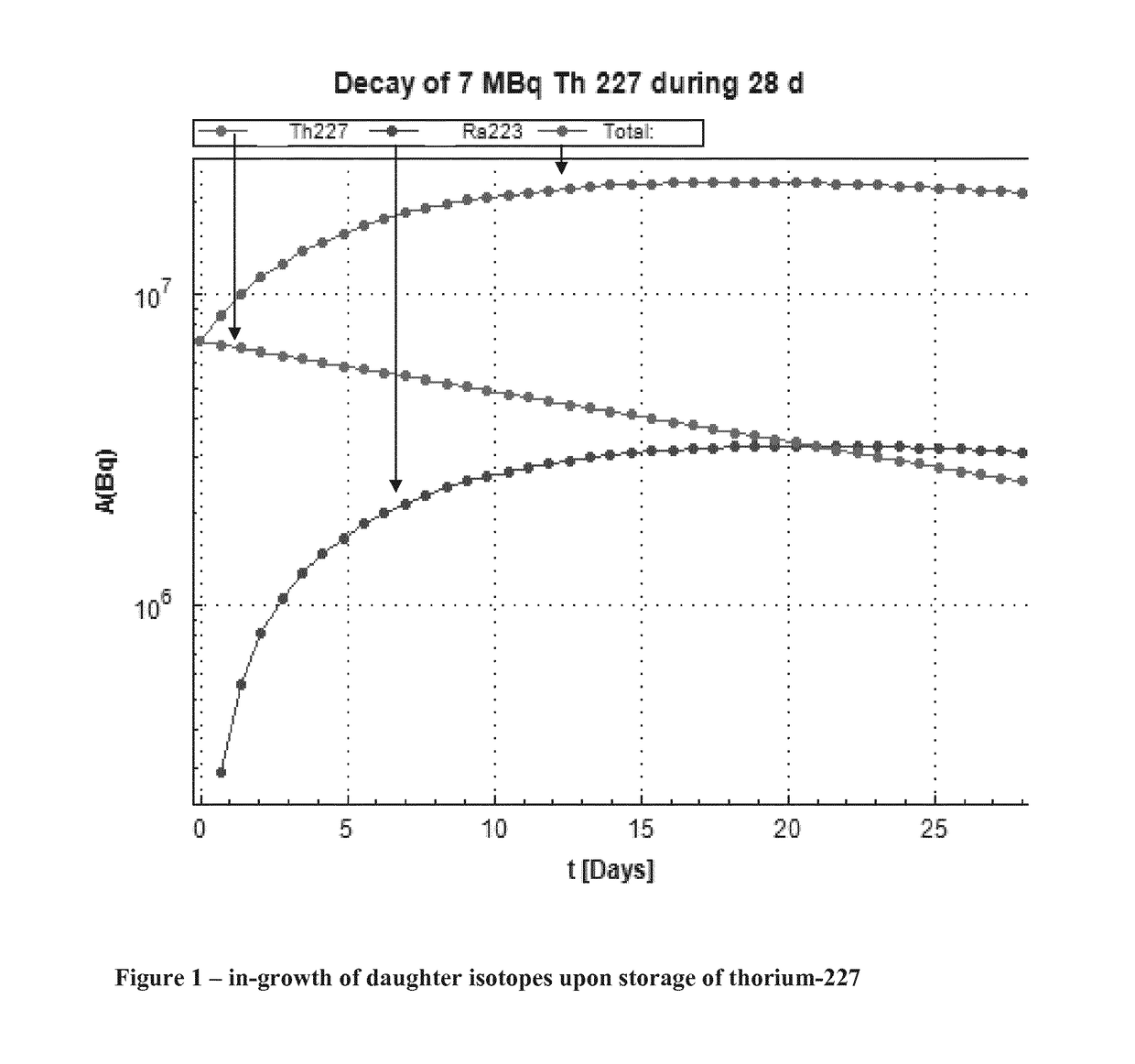
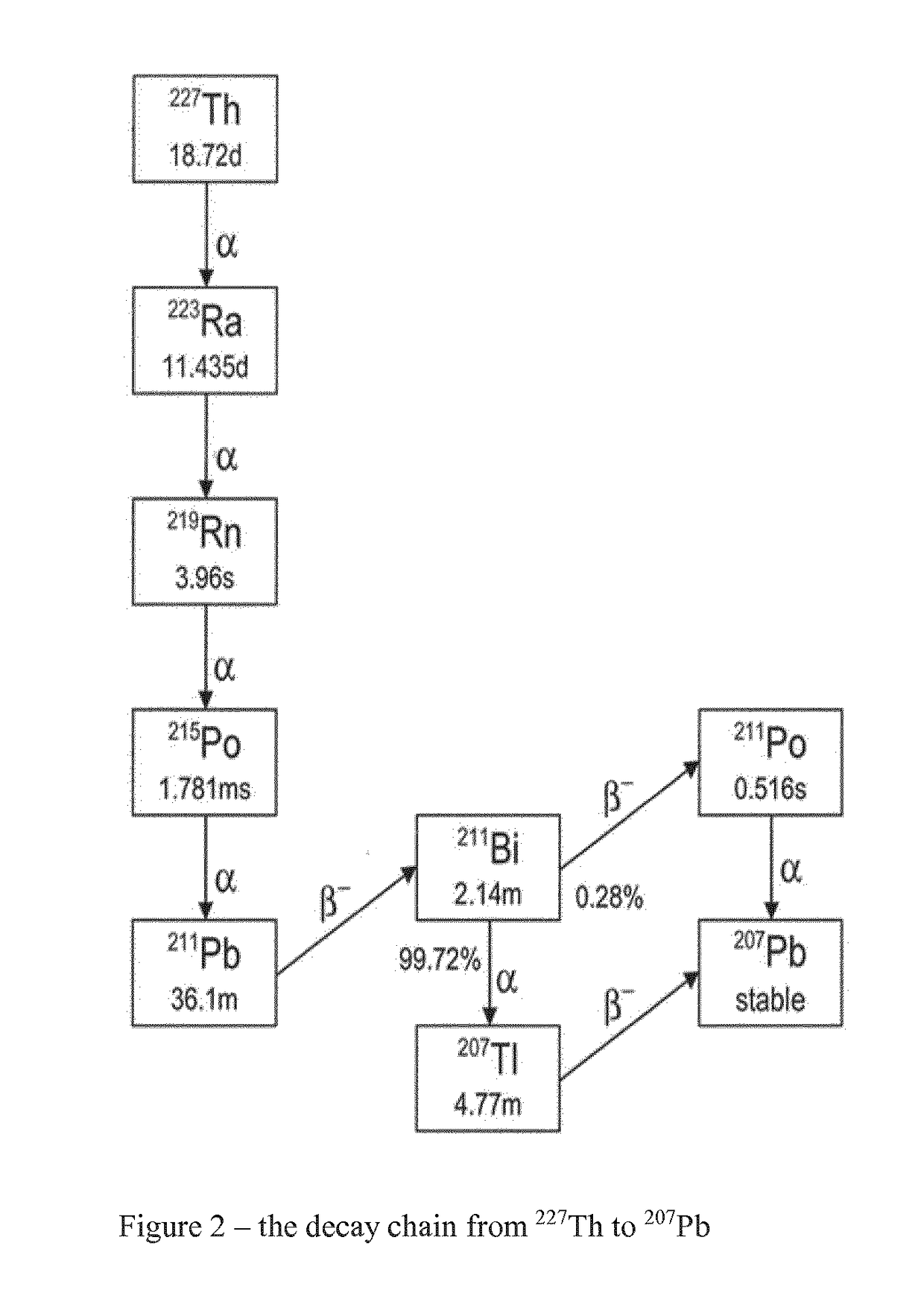
The invention provides a method for the purification of complexed 227Th from a mixture comprising complexed 227Th and 223Ra (complexed or in solution), said method comprising: i) preparing a first solution comprising a mixture of complexed 227Th ions and 223Ra ions in a first aqueous buffer; ii) loading said first solution onto a separation material; iii) eluting complexed 227Th from said separation material whereby to generate a second solution comprising complexed 227Th; iv) Optionally rinsing said separation material using a first aqueous washing medium; The invention additionally provides a purified 227Th solution, a pharmaceutical product and its use in treatment of disease such as cancer and a kit for generation of such a product.
Owner:BAYER AS
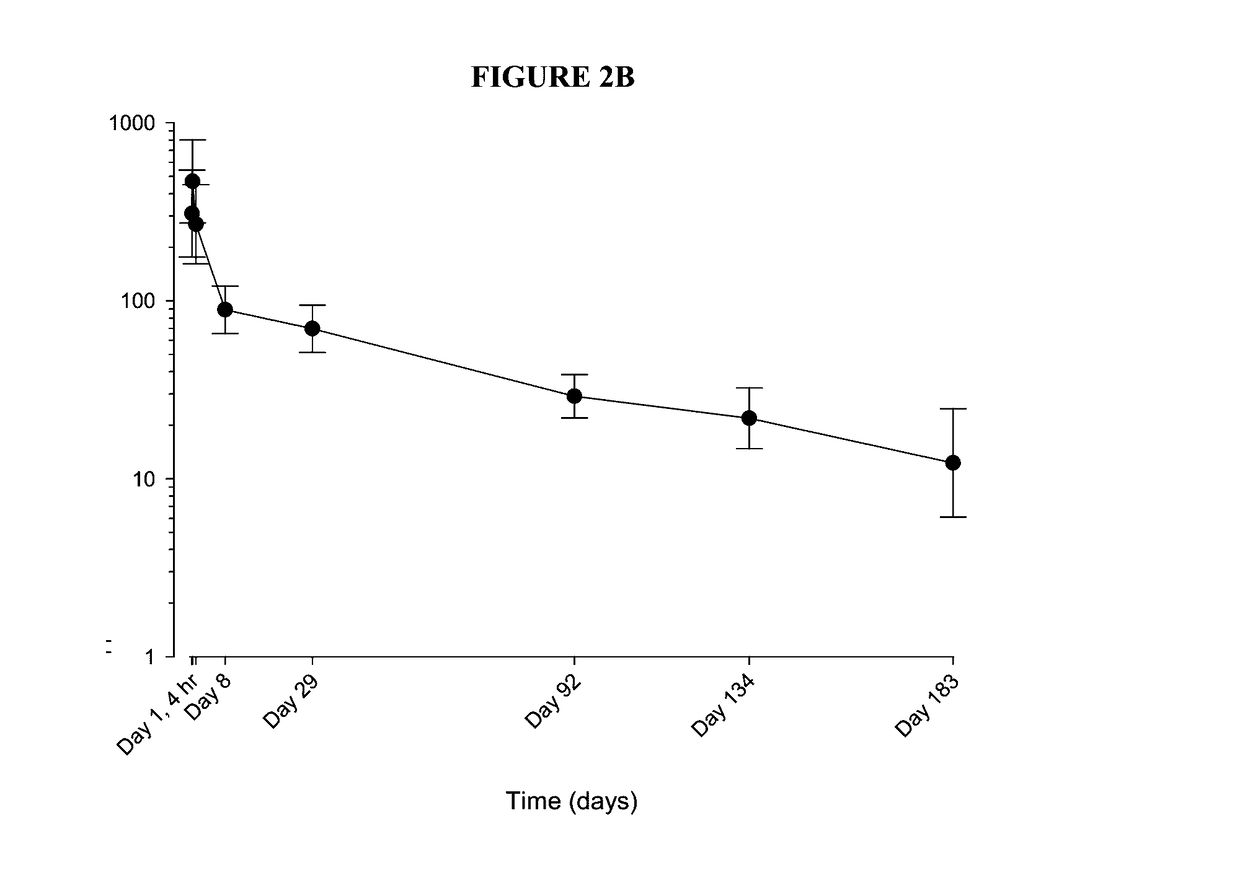
Corticosteroid formulations for maintaining corticosteroid synovial fluid concentrations
InactiveUS20170100411A1Good effectMaximize the effectOrganic active ingredientsSkeletal disorderFluticasone propionateCorticosteroid preparation
This invention relates to compositions and methods for achieving and maintaining maximal analgesic effect following intra-articular administration of corticosteroid formulations. The invention also describes extended release, e.g., controlled- or sustained-release corticosteroid formulations, including extended release, e.g., controlled- or sustained-release formulations of triamcinolone acetonide (TCA), fluticasone propionate, cortisol, ciclesonide (monopropionate), beclometasone diproprionate, dexamethasone, flunisolide, budesonide, desisobutyryl-ciclesonide, and / or mometasone furoate, that produce a maximal analgesic effect greater than the acute analgesic effect provided by standard corticosteroid suspensions, including non-extended release corticosteroid suspensions, and that are also associated with a clinically insignificant effect on endogenous cortisol production following administration, for example, intra-articular, intrathecal, epidural, intra-bursal, or other local administration.
Owner:FLEXION THERAPEUTICS
Application of radiofrequency catheter ablation system to treatment of essential hypertension
An application of a radiofrequency catheter ablation system to treatment of essential hypertension. The treatment process is under ultrasonic guidance, an electrode needle is made to penetrate into target tissue of a patient, electrification is conducted to start ablation, the needle is withdrawn or made to penetrate into the other side of the target tissue for target ablation after reaching ablation temperature and duration time, and overall ablation treatment is completed. The system can treat a secondary center for regulating the activity of a whole-body sympathetic system to reduce its activity, thus, the blood pressure level of a patient can be reduced, and fewer kinds and smaller dosage of antihypertensive drugs taken or ceased altogether. By treating the secondary center, the activity of the whole-body sympathetic system, insulin resistance and whole-body fibrosis is reduced, and other diseases characterized in activity abnormity of the sympathetic system might be treated too.
Owner:NANJING GUANGCI MEDICAL TECH
Perfusion device and method for operating same
ActiveUS11452802B2Side effectContainment leakOther blood circulation devicesDialysis systemsAllogeneic transfusionDialysis units
A negative-balanced isolated pelvic perfusion method, in which a drug is administered into the closed pelvis while keeping the volume of suction from the vein larger than that of injection into the artery, does not require allogeneic blood transfusion. A perfusion device is for recovering a liquid containing a drug and / or blood from a tube connecting to the inferior vena cava and for injecting the liquid obtained into a tube connecting to the artery, provided with a unit for closing the inside of the pelvis by including a unit for blocking the artery from the heart to the pelvis, a unit for blocking the inferior vena cava from the pelvis to the heart, and a unit for blocking a blood flow from the pelvis to the lower limbs. The perfusion device is provided with a pelvic perfusion unit equipped with a reservoir, an autotransfusion unit, and a dialysis unit.
Owner:KOSEI ADVANCE
Automatic bag making machine and bag making method thereof
PendingCN113681984AMeet different width requirementsSide effectBag making operationsPaper-makingHeat sealerEconomic benefits
The invention relates to the technical field of bag making machines, and discloses an automatic bag making machine and a bag making method thereof. The automatic bag making machine comprises a bottom plate and wound films, wherein a supporting plate is fixedly arranged on the upper surface of the bottom plate, two winding rollers are rotationally arranged on the outer surface of the supporting plate, and the two winding rollers are the upper film winding roller and the lower film winding roller correspondingly; and a stepping structure is arranged on the upper surface of the bottom plate, a heat sealing mechanism is arranged on the upper surface of the bottom plate, a trimming mechanism is arranged on the upper surface of the bottom plate, and a cutting mechanism is fixedly arranged on the upper surface of the bottom plate. According to the automatic bag making machine and the bag making method thereof, a sixth air cylinder is arranged, the sixth air cylinder drives a cutting tool to move downwards, and the wound films are cut one by one along a heat sealing trace of a second heat sealing strip according to the working requirement, so that packaging bags are formed; and the width and length of the produced packaging bags can be adjusted according to the working requirement, so that size adjustment is flexible and diversified, different working requirements can be met, the production range of the device is expanded, and the economic benefit of the device is improved.
Owner:无锡友迈智能装备有限公司
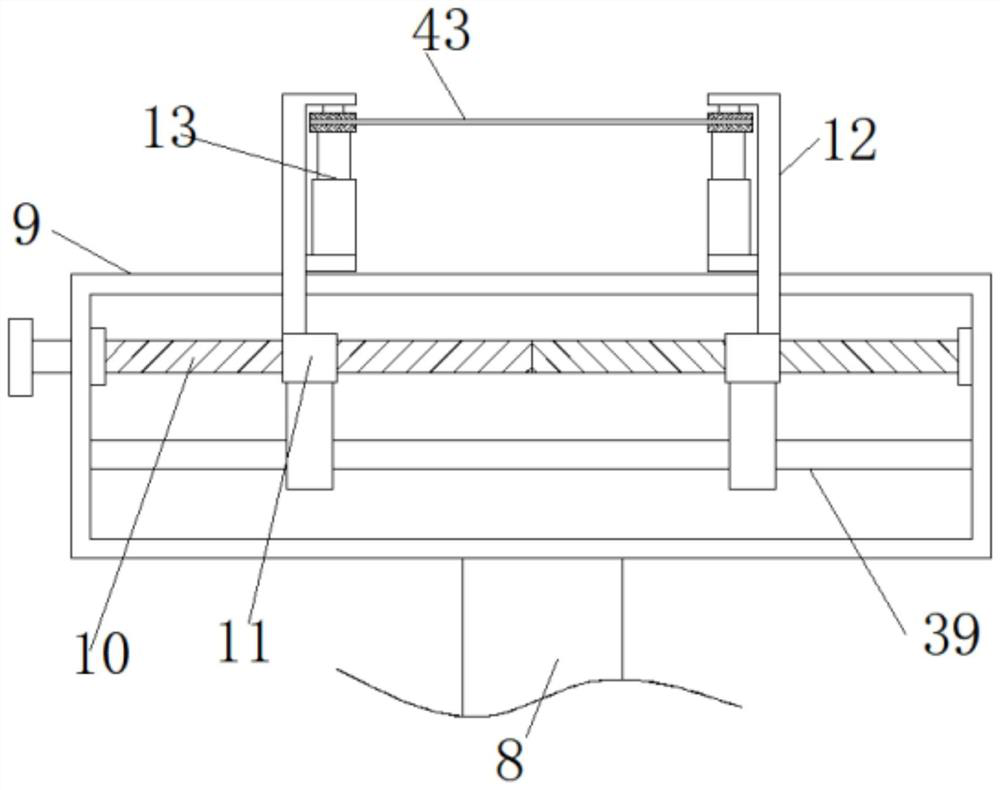
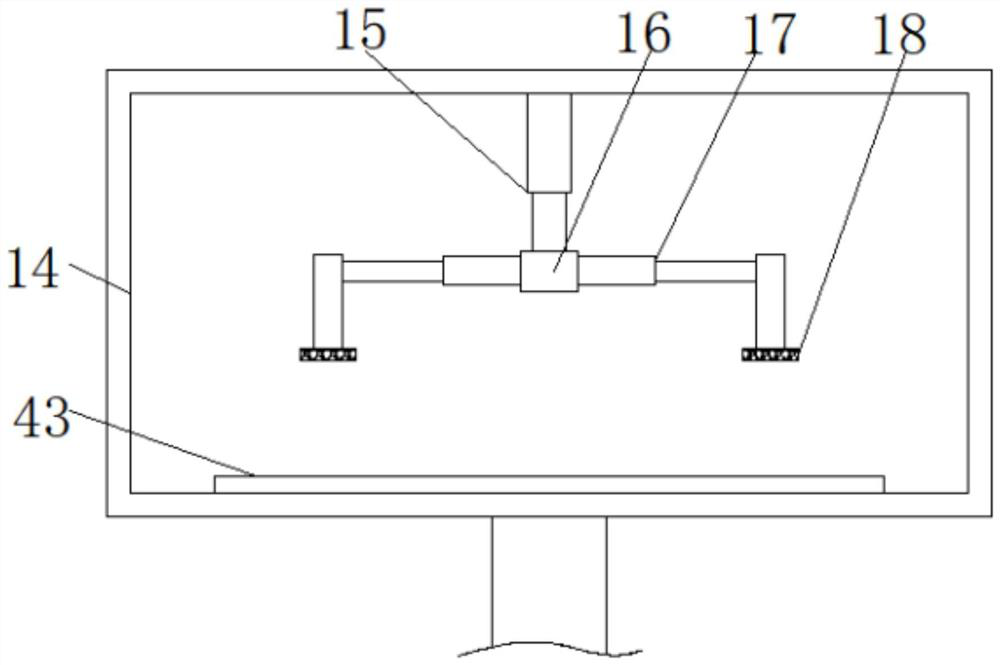
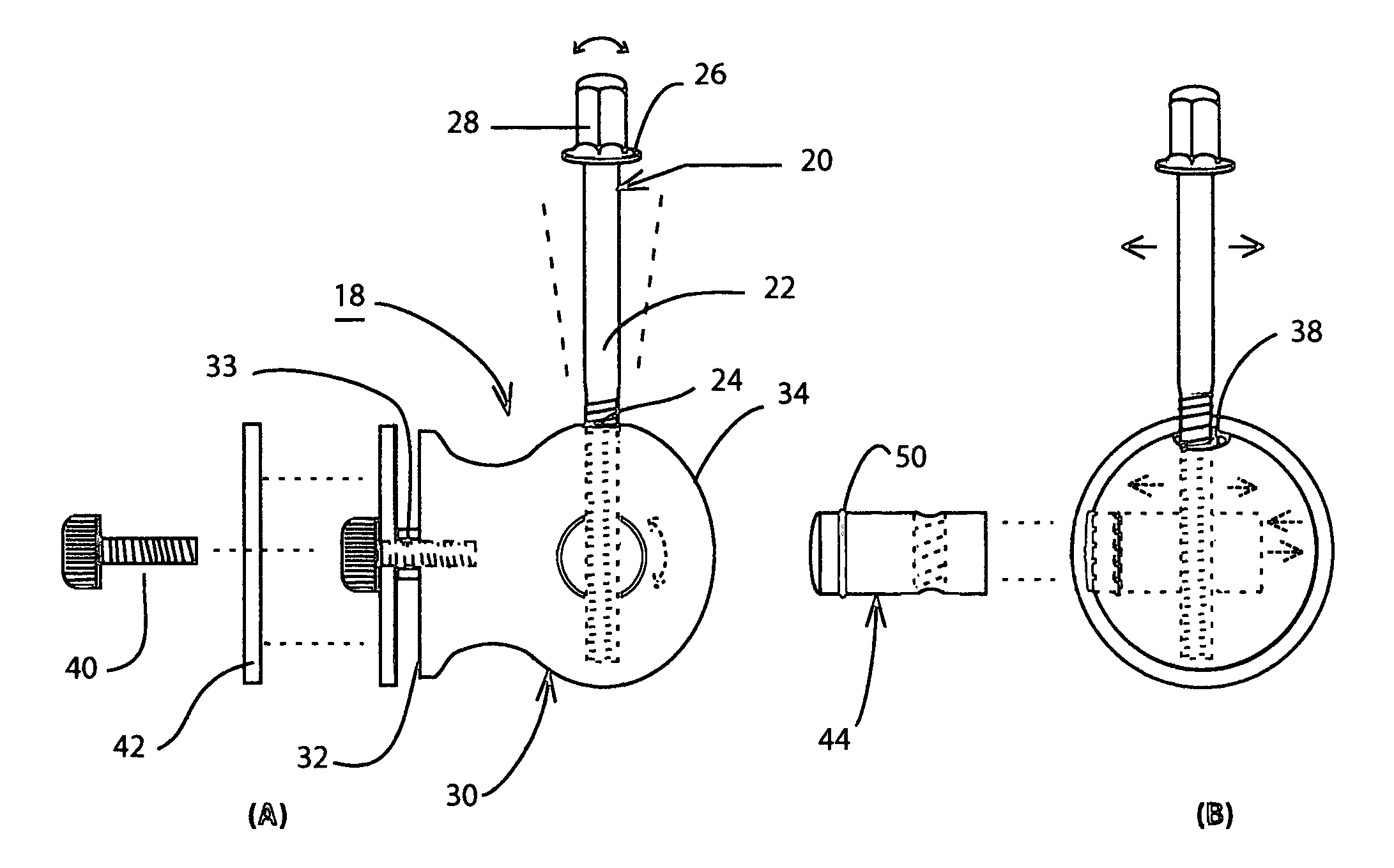
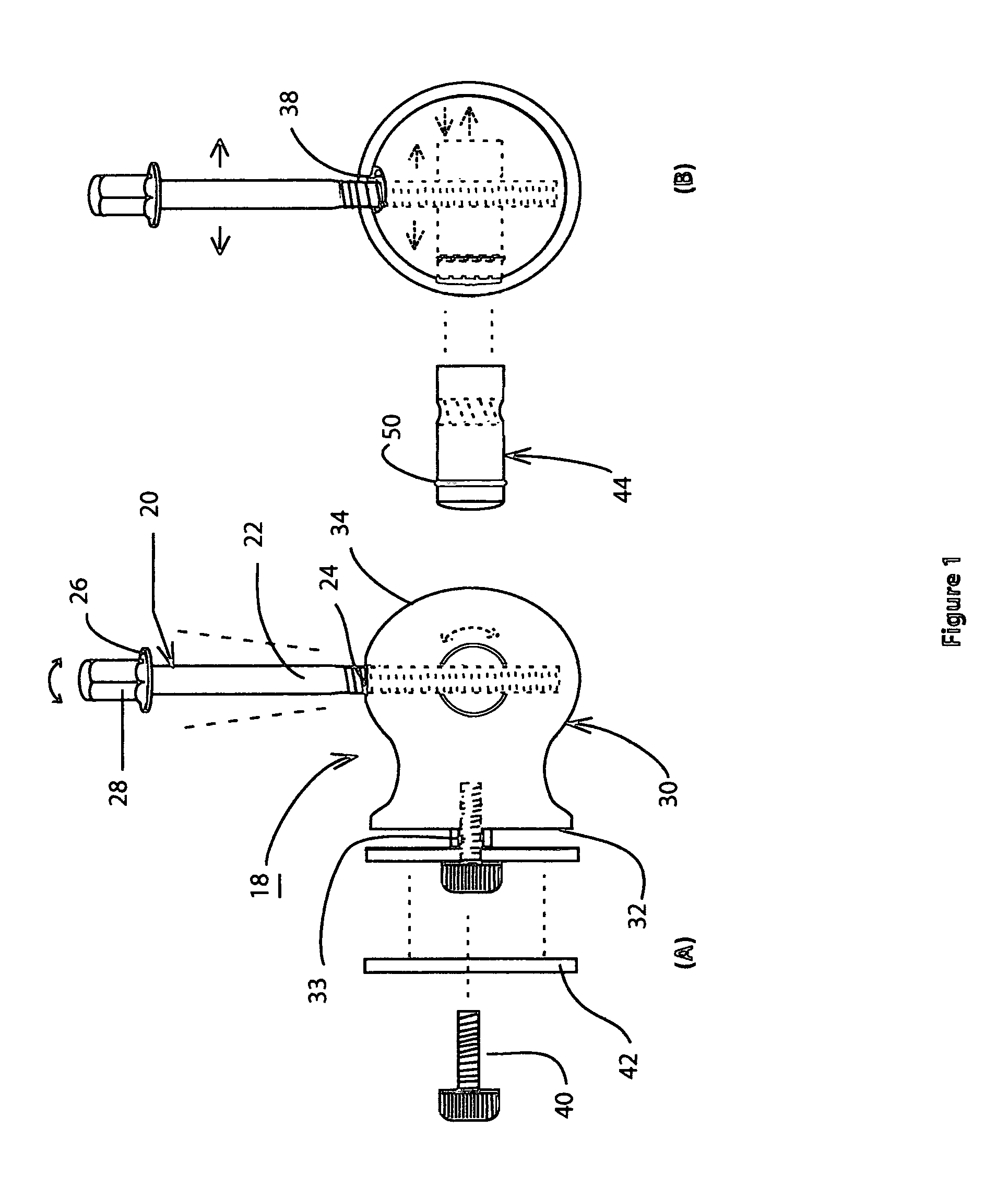
Drum head securement device
InactiveUS6956159B2Side effectMinimize side effectsPercussion musical instrumentsAutomatic musical instrumentsMechanical engineeringEngineering
A securement device for securing a drum head upon a drum shell or body through a tension rod, including a lug assembly that not only allows a downward pull on and hence tightening of the associated tension rod, but also provides for three additional parameters of adjustment of the position and aspect of the lower end of the tension rod relative to the associated drum shell.
Owner:EGO INDS
Treatment for dopaminergic disorders
ActiveUS20140309260A1Delay disease progressionSide effectBiocideAnimal repellantsOxidative enzymePharmacology
The present invention provides systems, compositions and methods for treatment of dopaminergic disorders (e.g., Parkinson's disease) using the combination of a first compound that inhibits a voltage-gated calcium channel of the type Cav1.3 (e.g., a dihydropyridine such as isradipine), and a second compound that is monoamine oxidase inhibitor and / or is a nitric oxide synthase inhibitor (e.g., rasagiline or derivative thereof).
Owner:NORTHWESTERN UNIV
Migraine remedy
InactiveUS20070065486A1Minor side effectsReduce frequencyBiocideCarbohydrate active ingredientsMigrainePharmacology
The invention relates to uses and methods of treating migraine with a plant extract that includes a therapeutically effective dose of a mixture of flavonoids rich in proanthocyanidis. Methods and uses are described to substantially prevent migraine from occurring; reduce the frequency of migraine or reduce the severity of migraine symptoms.
Owner:MIGCO +1
Treatment of Meningiomas Using Phenylbenzothiazole, Stilbene, Biphenylalkyne, or Pyridine Derivitives
ActiveUS20170196999A1Side effectLess successfulOrganic active ingredientsRadioactive preparation carriersPerylene derivativesBiological drugs
A method and composition for treating a meningioma in a subject are disclosed. The method includes the step of administering to the subject a therapeutically effective amount of a composition including a cytotoxic agent associated with a phenylbenzothiazole derivative or a stilbene derivative or a biphenylalkyne derivative that accumulates within meningiomas. In one version of the method, the phenylbenzothiazole derivative is a compound of formula (V):
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Topically active steroids for use in interstitial pulmonary fibrosis
ActiveUS9168263B2Reduce deliverySide effectOrganic active ingredientsRespiratory disorderMetaboliteInterstitial pulmonary fibrosis
Owner:SOLIGENIX INC
Combinations of FGFR4 inhibitors and bile acid sequestrants
ActiveUS11229643B2Side effectImprove the level ofAmine active ingredientsAntineoplastic agentsSide effectPharmaceutical drug
The present invention relates to a pharmaceutical combination comprising an FGFR4 inhibitor and a bile acid sequestrant, to the use of the pharmaceutical combination in the treatment of cancer, to the use of a bile acid sequestrant to reduce or mitigate side-effects associated with FGFR4 inhibition therapy.
Owner:NOVARTIS AG
Topically Active Steroids for Use in Interstitial Pulmonary Fibrosis
ActiveUS20110015166A1Reduce deliverySide effectOrganic active ingredientsRespiratory disorderMetaboliteInterstitial pulmonary fibrosis
Owner:SOLIGENIX INC
Topically Active Steroids for Use In Interstitial Pulmonary Fibrosis
ActiveUS20160106760A1Reduce deliverySide effectOrganic active ingredientsRespiratory disorderMetaboliteInterstitial pulmonary fibrosis
Owner:SOLIGENIX INC
Nerve growth factor activity potentiating agents
InactiveUS20050192343A1Side effectEliminate side effectsBiocideNervous disorderBULK ACTIVE INGREDIENTDisease cause
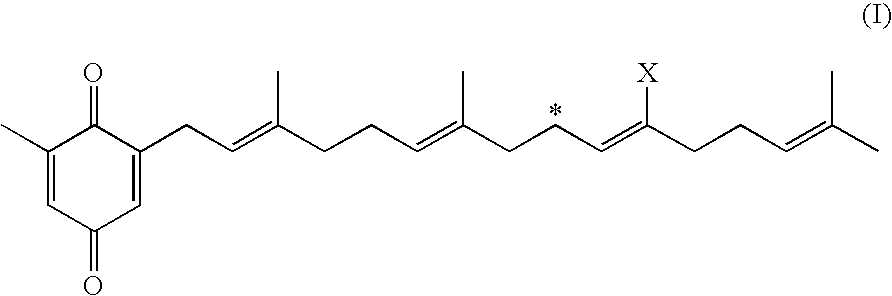
Agents potentiating nerve growth factor activity are provided for treating Alzheimer's disease which contain as the active ingredient dodecatrienoic acid derivatives represented by the general formula (I): wherein X represents hydrogen, CHO, COOH, COOR (wherein R represents an ester residue or an addition salt comprising an alkali metal salt or an organic acid salt group), provided that in case where X is COOH, it is bonded to the carbon atom at the position marked with * so as to form a lactam O═C—O—.
Owner:GOTO TAKESHI +3
Pharmaceutical composition and healthy food composition with Lactobacillus sp. KCCM 11826P for preventing or treating hyperphosphatemia in chronic kidney disease
ActiveUS11123385B2Easy to optimizeEffective controlBacteriaMicroorganism based processesBiotechnologyLactobacillus
Provided are a functional food or pharmaceutical composition or a method for preventing, alleviating or treating hyperphosphatemia and chronic kidney disease and treating chronic kidney disease using the composition comprising Lactobacillus sp. KCCM 11826P having excellent phosphorus-absorbing ability.
Owner:KOREA FOOD RES INST +1
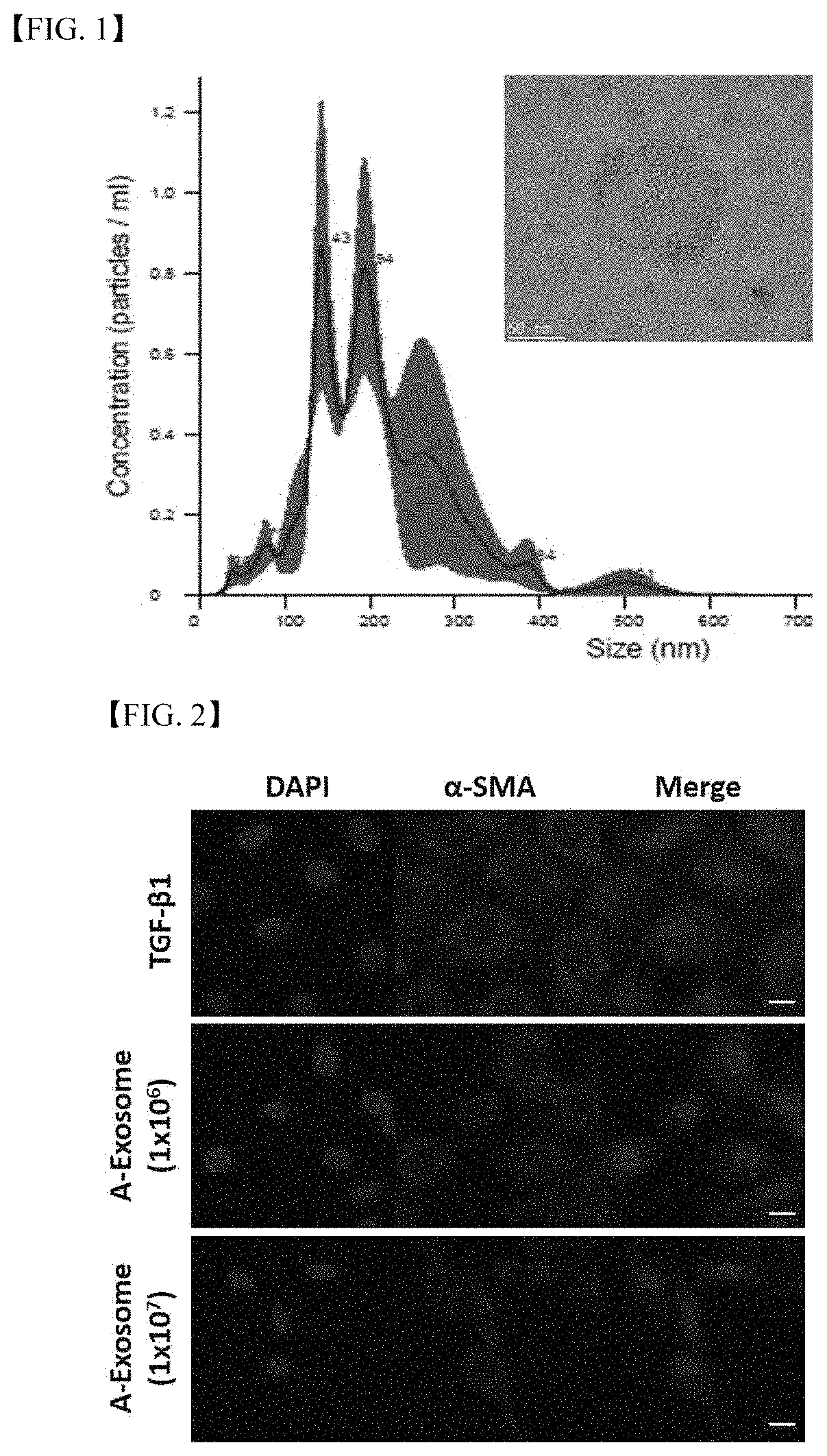
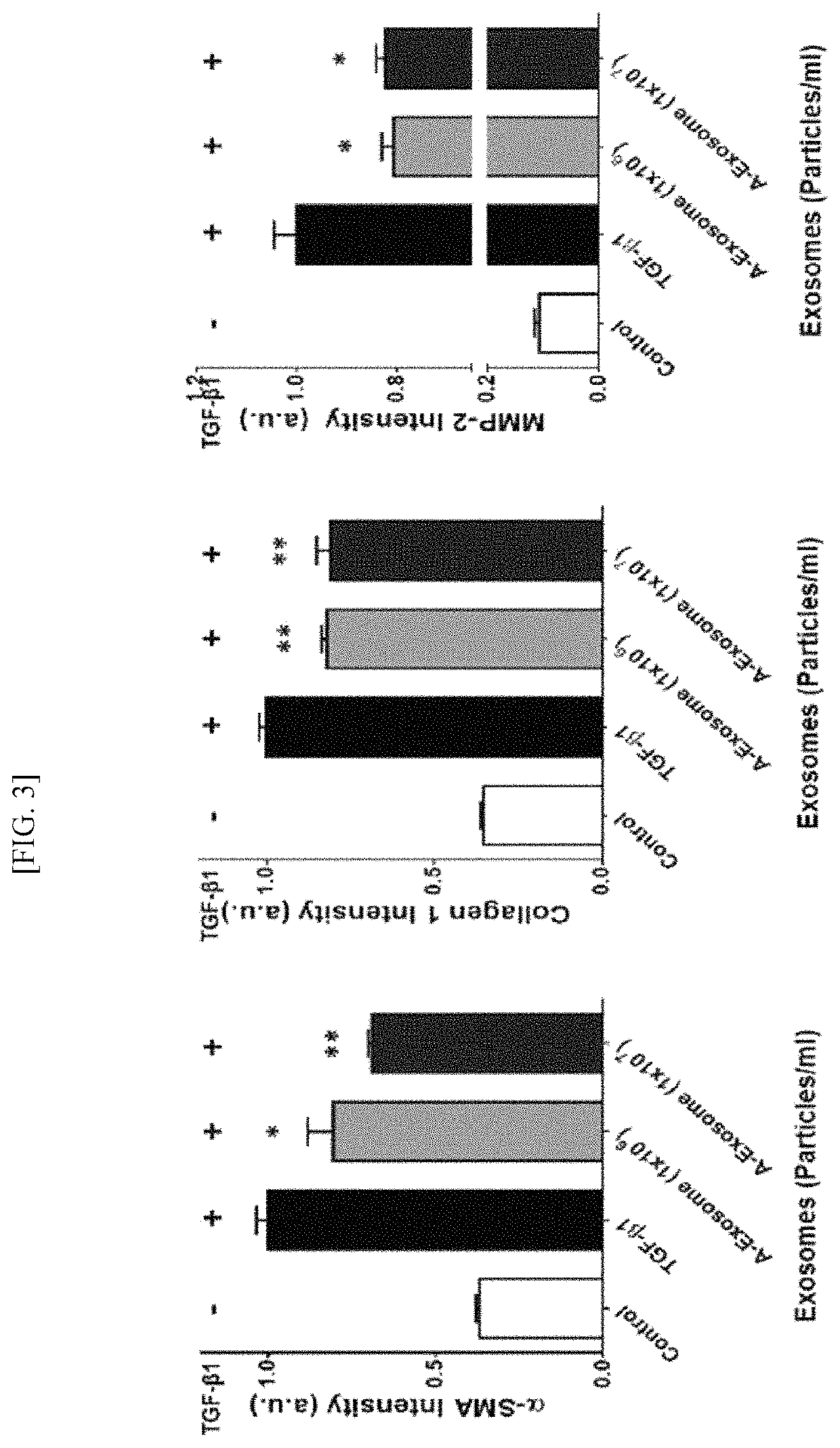
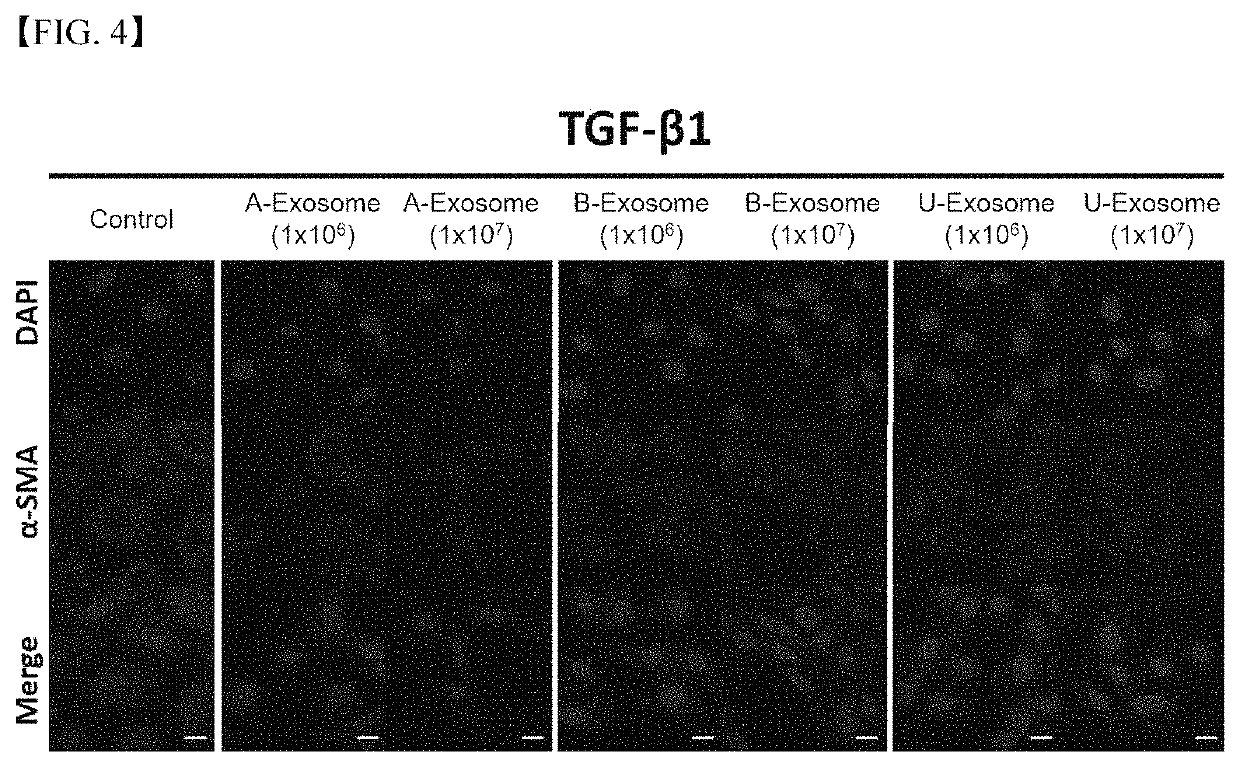
Treating liver fibrosis using adipose stem cell-derived exosomes as active ingredient
ActiveUS11241459B2Good biocompatibilitySuperb absorption rateDigestive systemUnknown materialsExosomeLiver fibrosis
The present embodiment relates to a composition containing human adipose stem cell-derived exosomes as an active ingredient for preventing, alleviating or treating liver fibrosis. The adipose stem cell-derived exosomes according to the present embodiment carry genetic information, protein, growth factor and the like for treating liver fibrosis, and have shown excellent results in treating liver fibrosis in animal models therefor, and, being a cell-derived substance, exosomes have the advantages of being highly biocompatible and having a superb absorption rate.
Owner:EXOSTEMTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com