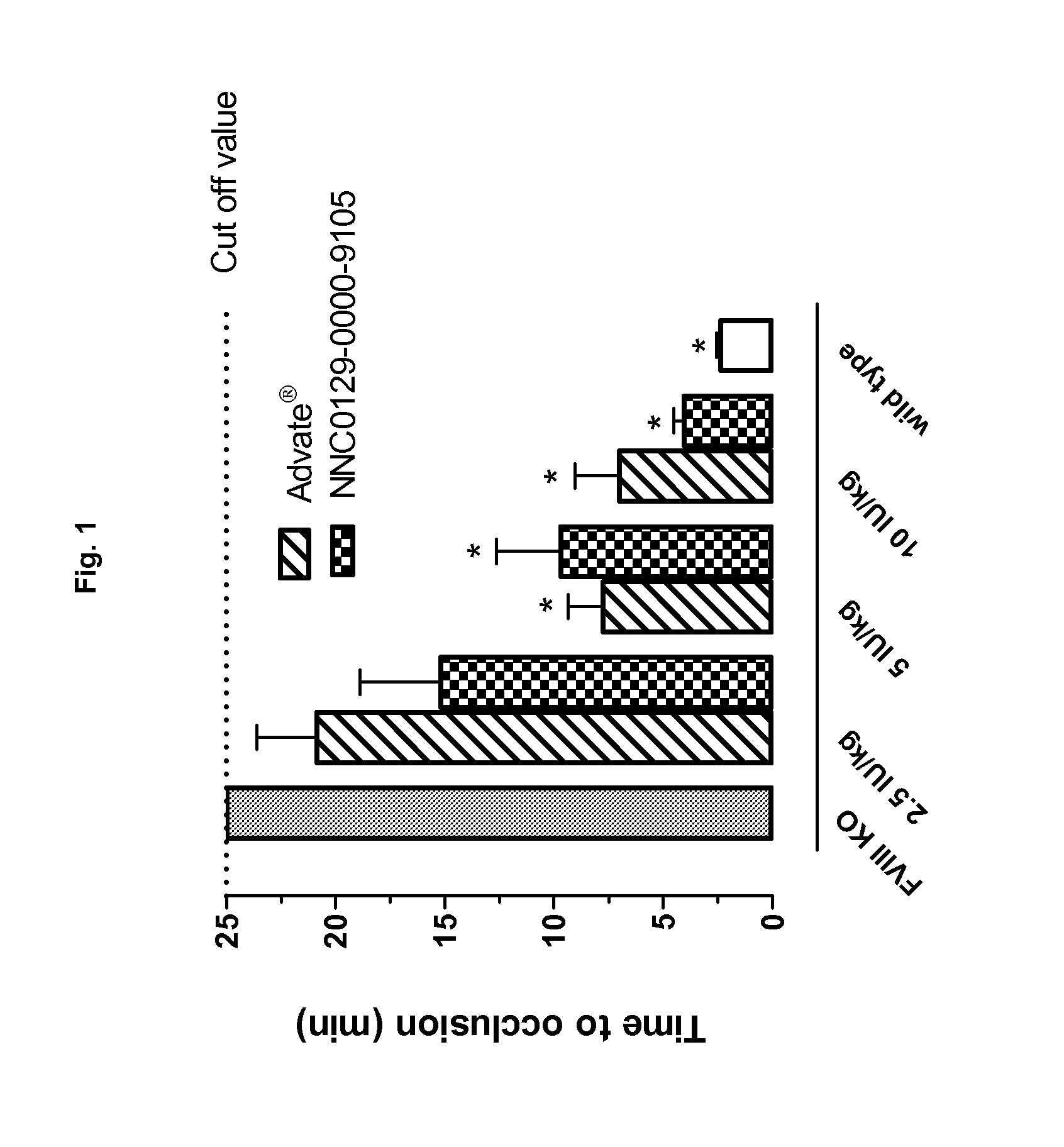
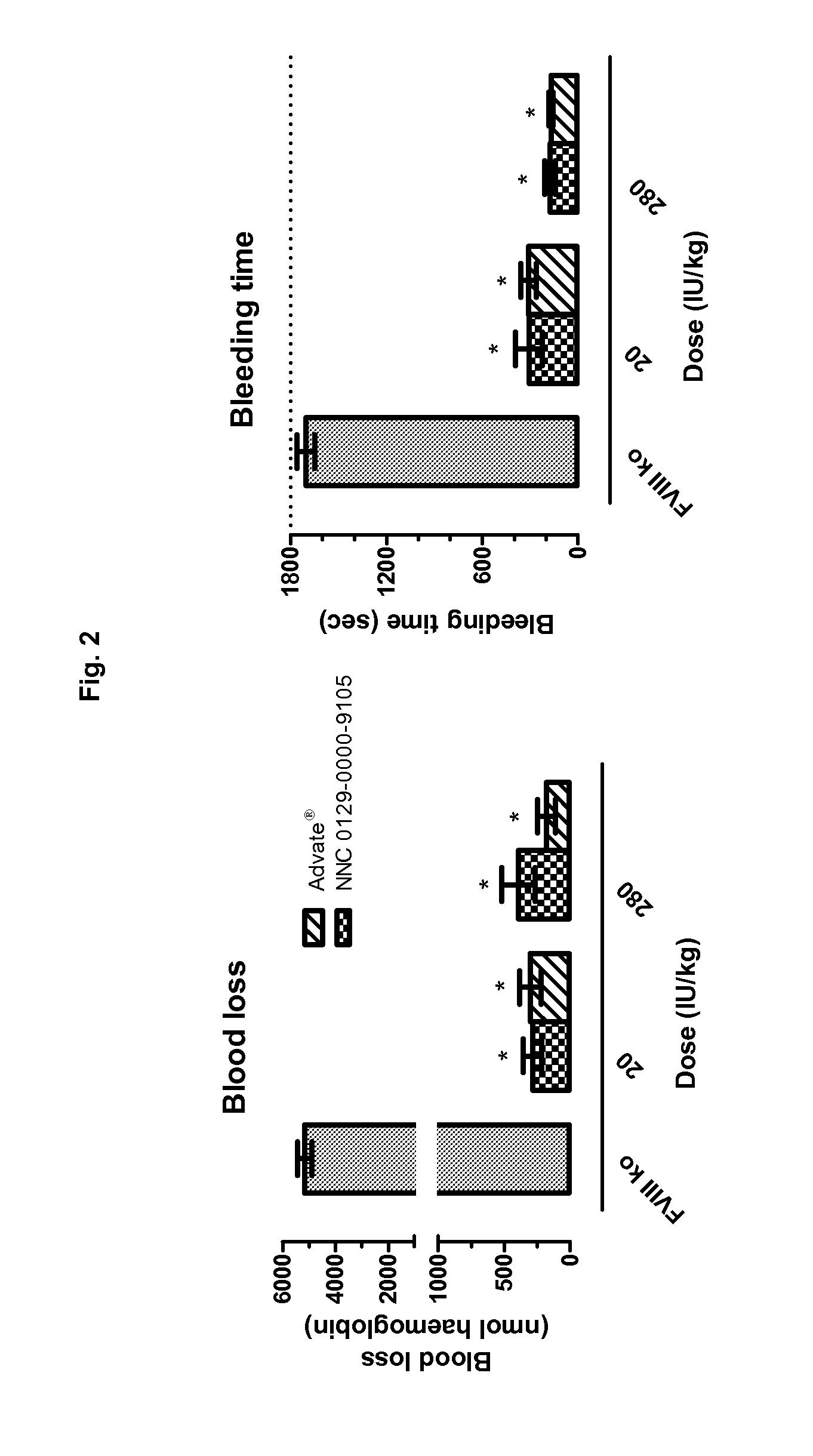
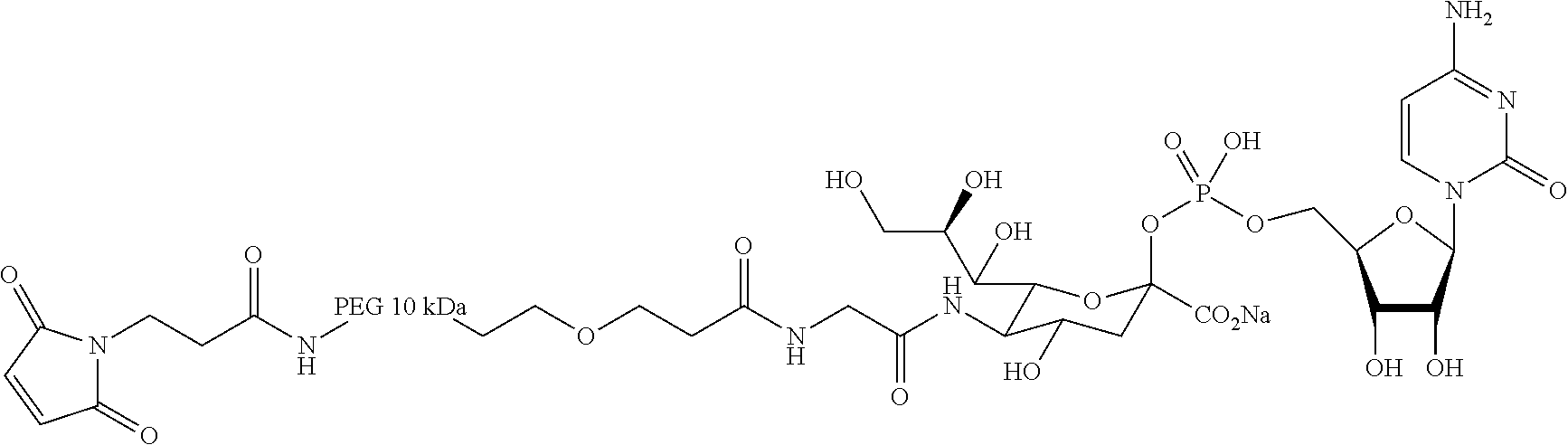
Factor VIII Molecules With Reduced VWF Binding
a technology molecule, applied in the field of recombinant factor viii (fviii) molecules, can solve the problems of affecting the quality of iv, unstable clot, and significant inconvenience and/or pain of many people, especially children and young peopl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Recombinant B Domain Truncated O-glycosylated Factor VIII and Varints Thereof, e.g., Factor VIII (Y1680F) or Factor VIII (Y1680C)
Cell Line and Culture Process
[0081]Using Factor VIII cDNA, a mammalian expression plasmid was constructed. The plasmids encodes a B-domain deleted Factor VIII comprising the Y1680F mutation, the Factor VIII heavy chain comprising amino acid 1-740 of full length human Factor VIII, and Factor VIII light chain comprising amino acid 1649-2332 of full length human Factor VIII. The heavy and light chain sequences are connected by a 21 amino acid linker (SFSQNSRHPSQNPPVLKRHQR—SEQ ID NO 4) comprising the sequence of amino acid 741-750 and 1638-1648 of full length human Factor VIII. FVIII variants comprising the linker defined in SEQ ID NO 4 may also herein be referred to as “N8”. The Factor VIII amino acid sequence encoded by this plasmid is as set forth in SEQ ID NO 1 (wt), SEQ ID NO 2 (Y1680F), SEQ ID NO 3 (Y1680C)
[0082]Chinese hamster ovary (CHO) ...
example 2
Procedure for PEGylation of Recombinant O-glycosylated Factor VIII
[0090]The recombinant Factor VIII molecules obtained in Example 1 are conjugated with polyethylenglycol (PEG) using the following procedure:
[0091]For the glycoPEGylation reaction to be efficient a FVIII concentration >5mg / ml is required. Since FVIII is not normally soluble at the concentration a screening of selected buffer compositions was conducted (see table 1). Based on these considerations a buffer containing 50 mM MES, 50 mM CaCl2, 150 mM NaCl, 20% glycerol, pH 6.0 was found to be a suitable reaction buffer.
TABLE 1Evaluation of impact of reaction conditions on FVIIIsolubility and aggregation.Reaction buffer compositionPrecipitate% Aggregate10 mM Histidine, 260 mM Glycine, 1%YESn.d.Sucrose, 10 mM CaCl250 mM HEPES, 10 mM CaCl2, 150 mMYESn.d.NaCl, pH 7;50 mM MES, 10 mM CaCl2, 150 mM NaCl,YESn.d.pH 6.050 mM MES, 50 mM CaCl2, 150 mM NaCl,NO8pH 6.050 mM MES, 50 mM CaCl2, 150 mM NaCl,NO510% glycerol, pH 6.050 mM MES, 5...
example 3
Pegylation of Y1680C with Peg-30K-Maleimide (ref. US2006 / 0115876 A1)
Reagents:
[0104]1) BDD-FVIII N8-Y1680C (MW 178,000), 1200 μl, conc. 80 μg / ml, 96 μg, 0.54 nmol in buffer 20 mM imidazole, +10 mM CaCl2, +0.02% Tween 80, +1 M NaCl, 1 M gGlycerol, pH 7.3[0105]2) Triscarboxyethylphosphine (TCEP, MW 287): 700 eq; 0.0315 μmMol; 9 μg. 1 mg TCEP was dissolved in 1 ml of buffer 20 mM limidazol, 10 mM CaCl2, 0.02% Tween 80, 1 M Gglycerol, pH 7.3, 1 M NaCl. 109 ul of this solution was used.[0106]3) 30 kDa PEG-maleimid (Sunbright Me-300Ma from NOF Corp., MW 29300), 10 eq., 180 pg. 4.8 mg 30 kDa PEG-maleimid was dissolved in 2.4 ml of buffer 20 mM limidazol, 10 mM CaCl2, 0.02% Tween 80, 1 M Gglycerol, pH 7.3, 1 M NaCl. 90 uμl of this solution was used.
[0107]Buffers used for VivaP pure spin column (strong anion exchange) and Pro-spin (spin columns):
Buffer A: 20 mM Imidazol, 10 mM CaCl2CaCl2, 0.02% Tween 80, 1 M Glycerol, pH 7.3
Buffer B: 20 mM Imidazol, 10 mM CaCl2CaCl2, 0.02% Tween 80, 1 M Gly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com