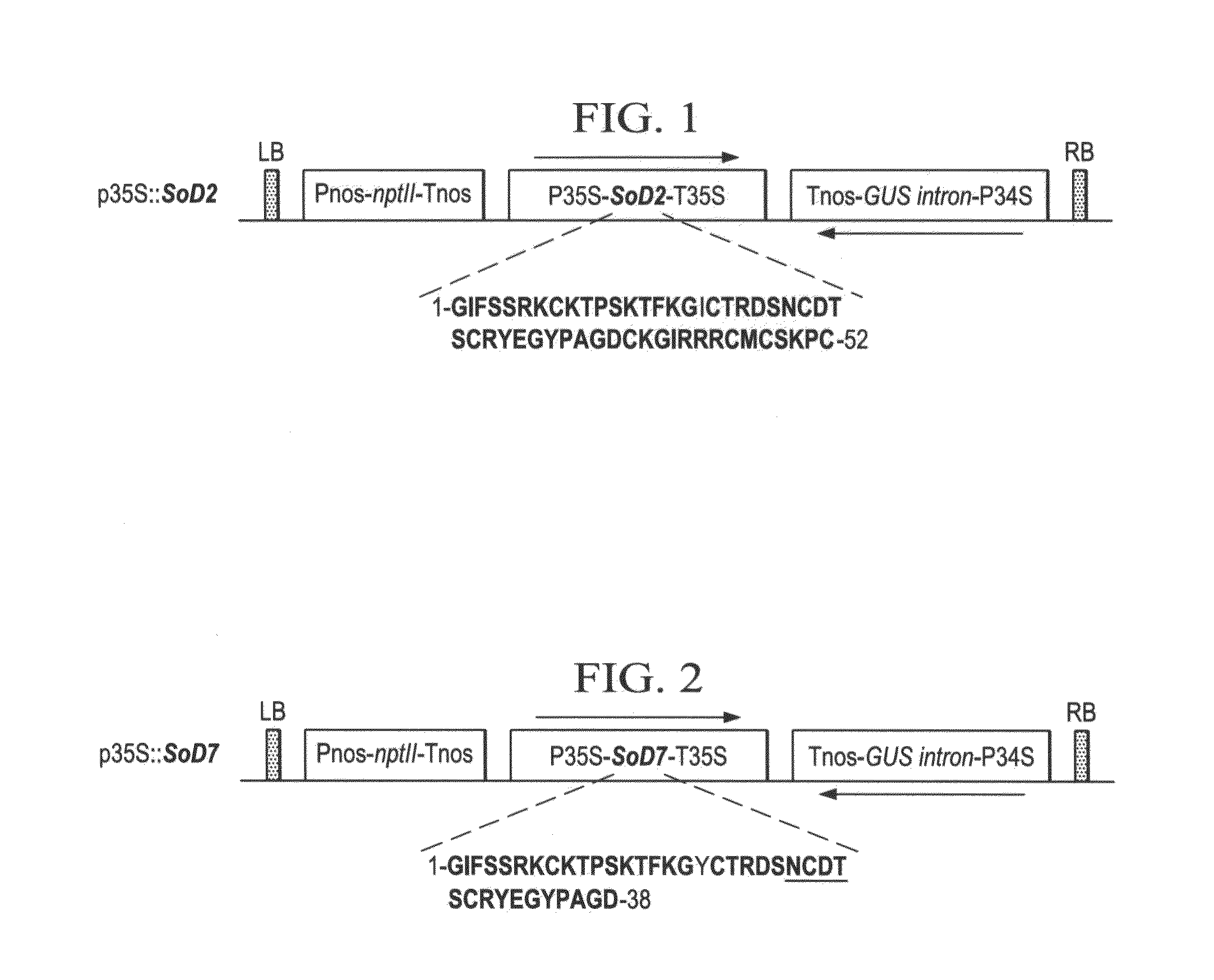
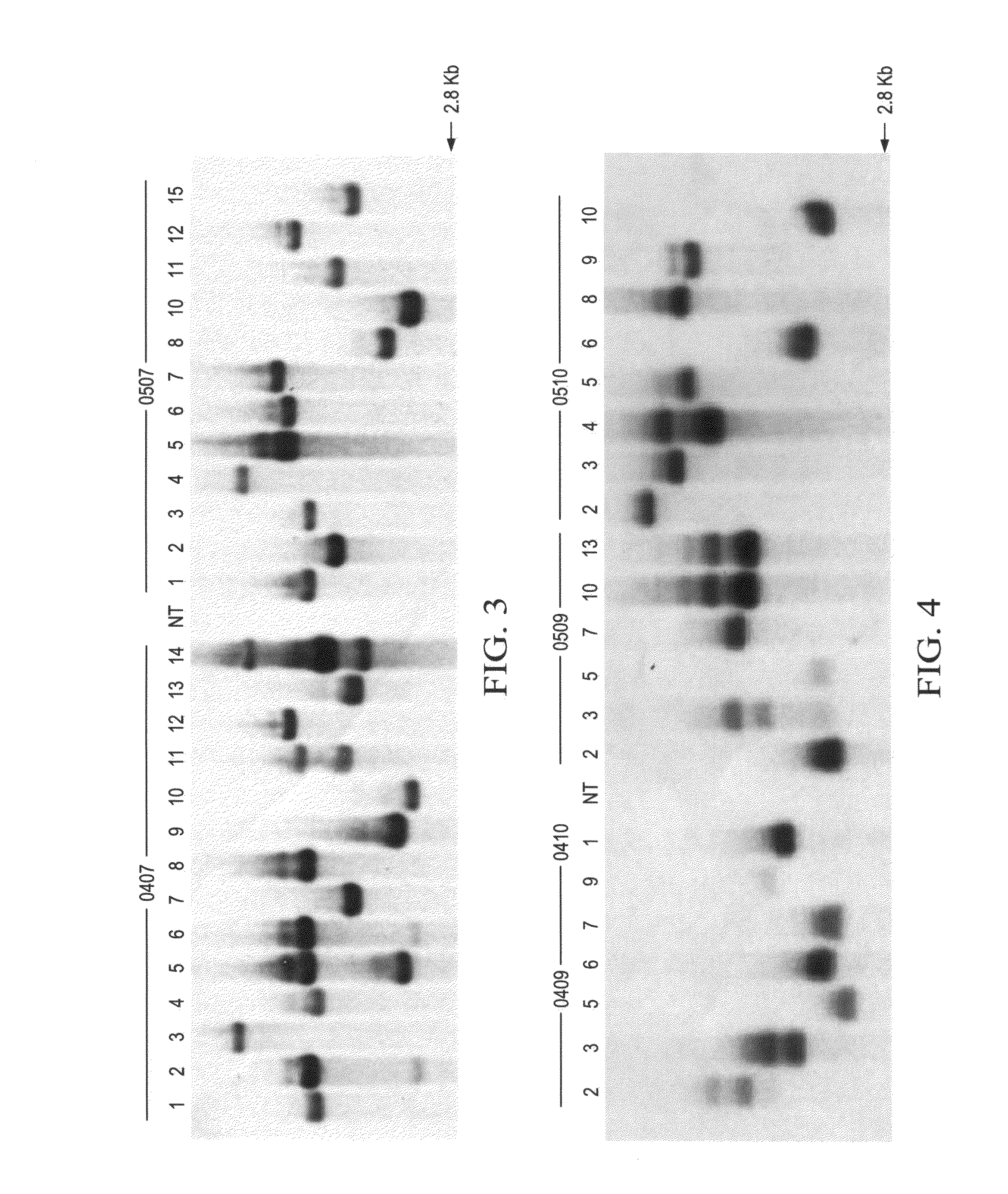
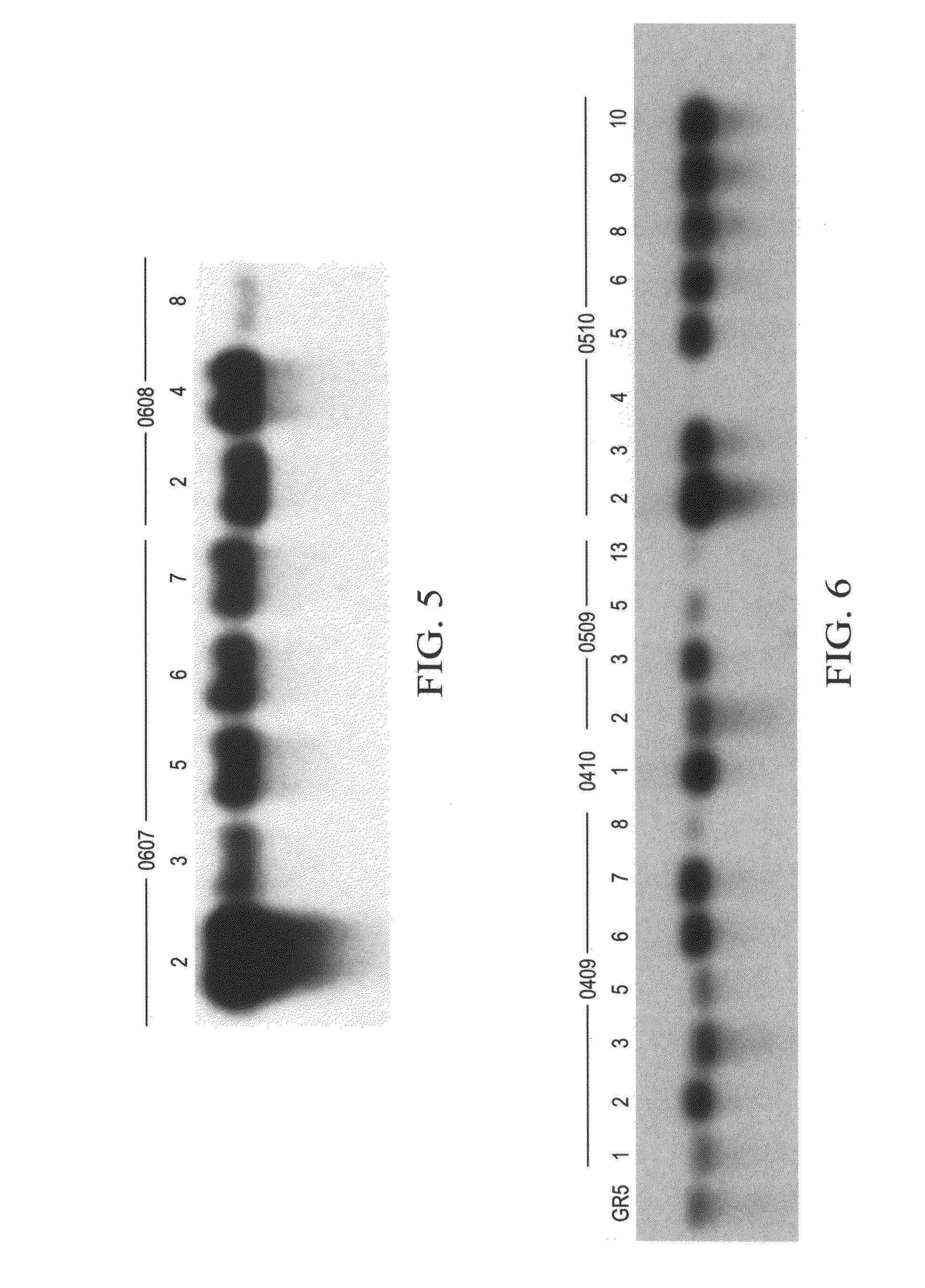
Pathogen resistant citrus compositions, organisms, systems, and methods
a technology of organisms and compositions, applied in the field of pathogen resistant citrus compositions, organisms, systems, and methods, can solve the problems of long life cycle, complex reproductive biology, and inability to find genetic resistance to these microbial pathogens within the citrus genus, and achieve the effect of preventing the spread of pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plant Material
[0102]Plant materials (e.g., Citrus sinensis) were generally prepared for transformation as described by Yang et al., Plant Cell Reports (2000) 19:1203 et seq.
example 2
Plasmid Construction and Bacterial Strains
[0103]Plasmid construction and bacterial strains were generally performed as described by Yang et al., Plant Cell Reports (2000) 19:1203 et seq.
example 3
Agrobacterium Co-Culture and Plant Transformation
[0104]Agrobacterium co-culture and plant transformation were generally performed as described by Yang et al., Plant Cell Reports (2000) 19:1203 et seq.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com