Antibody formulations and methods
an antibody and formulation technology, applied in the field of immunology and medicine, can solve the problems of uncharacterized organ dysfunction, no currently approved treatment for al amyloidosis that directly targets potentially toxic forms,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selection of Humanized 2A4 for the Treatment of AL Amyloidosis
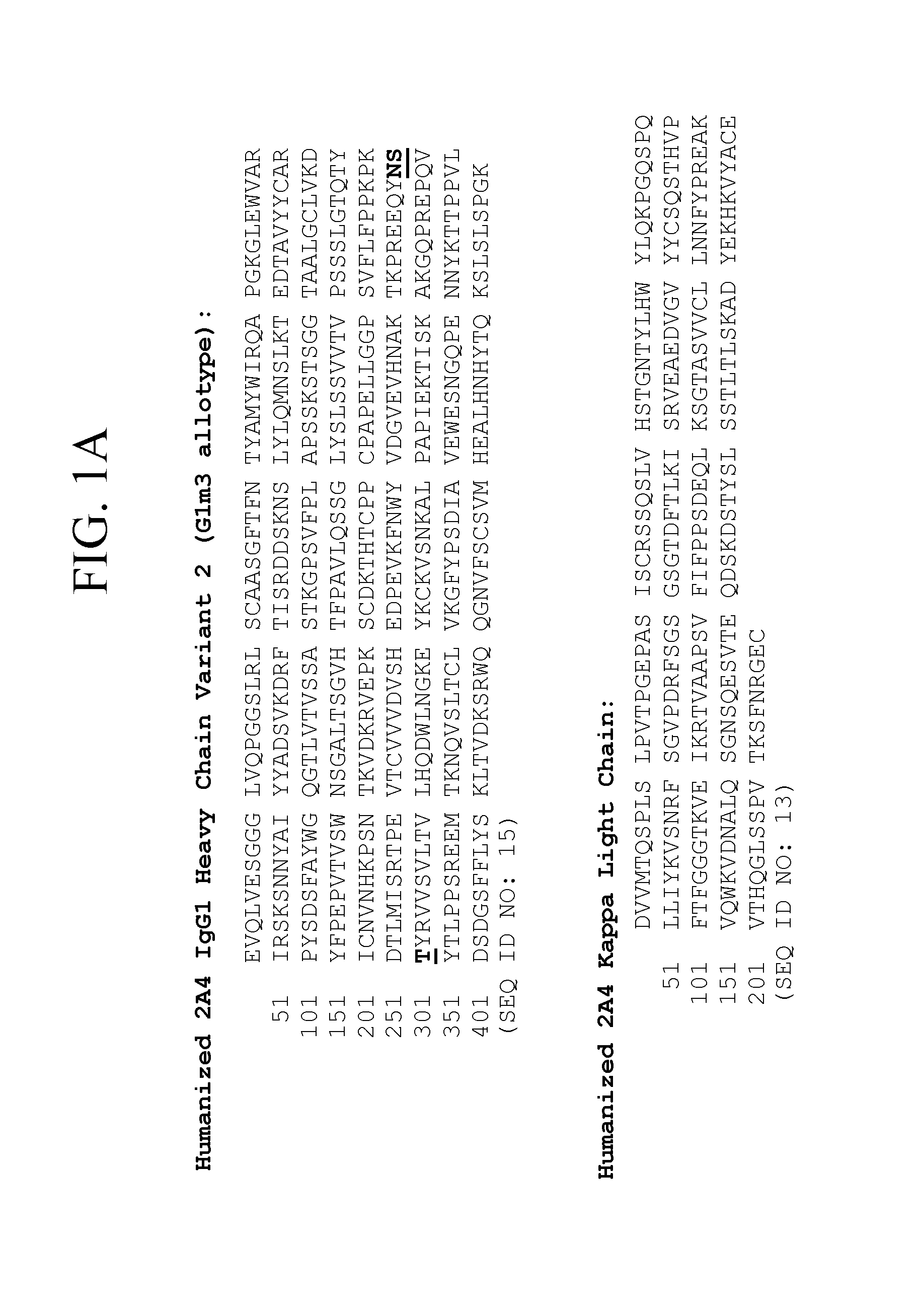
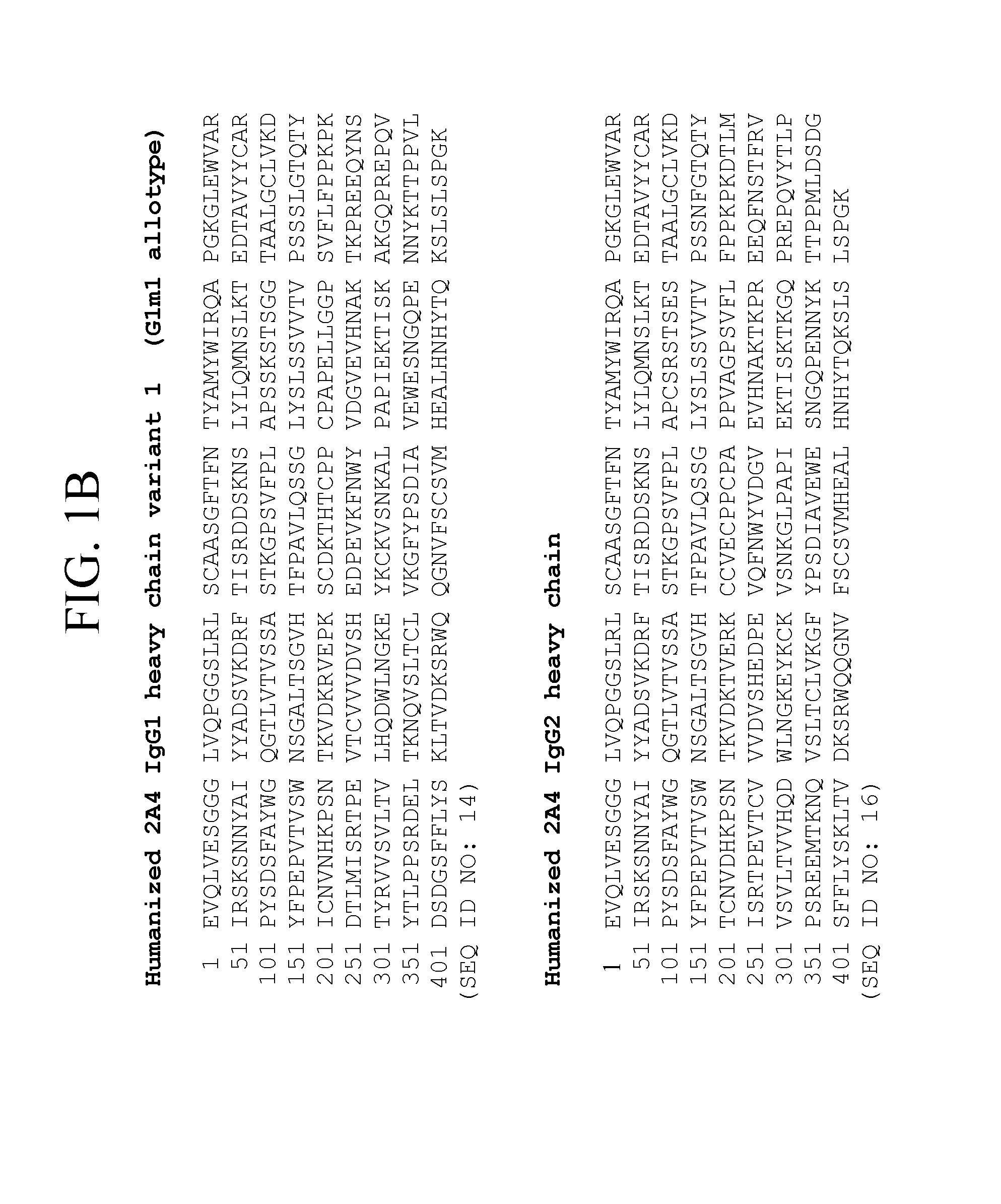
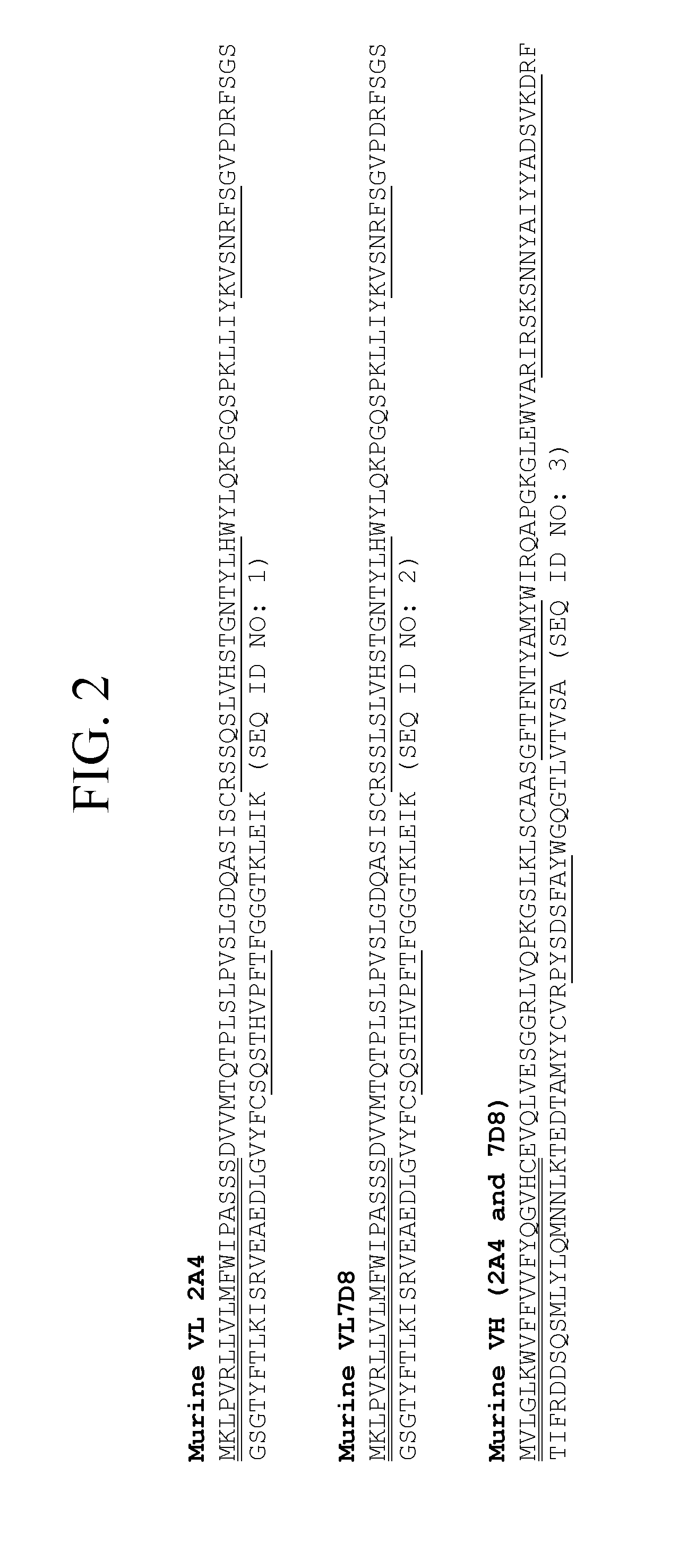
[0096]An IgG1, kappa isotype antibody was prepared, which is a humanized version of murine antibody 2A4. The light chain and heavy chain sequences of representative humanized 2A4 antibodies are set forth in FIGS. 1A-1B and 3. Nucleic acids encoding the particular humanized 2A4 antibody version 3, which amino acid sequences are shown in FIG. 3, are depicted in FIGS. 4A-4B.
[0097]The parent monoclonal 2A4 antibody is directed against a neo-carboxy terminal epitope of human serum Amyloid A (sAA), resulting from cleavage of the native sAA molecule at amino acid residue 76. The murine antibody does not cross-react with IgGs or free light chain (LC) and it has shown broad isotype recognition of patient derived AL amyloid samples examined to date. 2A4 recognizes multiple forms of AL light chain amyloid including soluble multimer and insoluble deposits. In addition, the antibody has been shown to promote regression of amyloidoma i...
example 2
Dose Determination for Humanized 2A4 Antibody
[0098]Nonclinical studies in the TRIAD mouse model and the cynomolgus monkey have utilized doses of 4 and 40 mg / kg in the mouse and 10, 50, and 100 mg / kg in the monkey. Conversion to the Human Equivalent Dose (HED) on a mg / kg basis (most appropriate conversion for monoclonal antibodies due to their restriction to the vascular space) gives HEDs of 0.32 and 3.2 for the mouse and 3.2, 16, and 32 for the monkey. Based on currently available data, the NOAEL in both species is expected to be the highest dose administered. Using a mouse HED (most sensitive species due to dosing limitations) of 3.2 and a 10× safety factor, the MRSD for first in man dosing would be approximately 0.32 mg / kg. Based upon animal studies, administration to humans is begun with a dose of 0.5 mg / kg.
example 3
Preparation of the Expression Vector
[0099]For generation of the final h2A4 IgG1 HC vector the variable region of the heavy chain was isolated by PCR using the plasmid CET1019AS-hygro-h2A4VH3-Sce 4.23.07 as template. Primers used for the amplification introduced at the 5′ end of the fragments an MfeI restriction site and at the 3′ end a BlpI restriction site for subcloning. The variable region was cloned into the MfeI and BamHI digested eukaryotic expression vector pBI-61, which contains the genomic constant regions of human IgG1 of G1m(3) allotype. The resulting recombinant expression vector pBI-61 / 2A4 IgG 1-REM is 9,015 base pairs in size and carries the selectable marker dihydrofolate reductase (DHFR) from hamster under the control of the DHFR promoter and polyadenylation signal. This vector also contains the beta-lactamase gene for selection in E. coli as well as origins of replication for E. coli (ColE1 ori), SV40 (SV40 ori) and filamentous phage f1 (f1 ori). Expression of the H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com