Colorectal cancer associated circulating nucleic acid biomarkers
a technology of circulating nucleic acids and colon cancer, which is applied in the field of colon cancer associated circulating nucleic acid biomarkers, can solve problems such as withdrawal from various testing methods, and achieve the effect of increasing the likelihood of the patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Colorectal Cancer-Associate CNA Study Samples
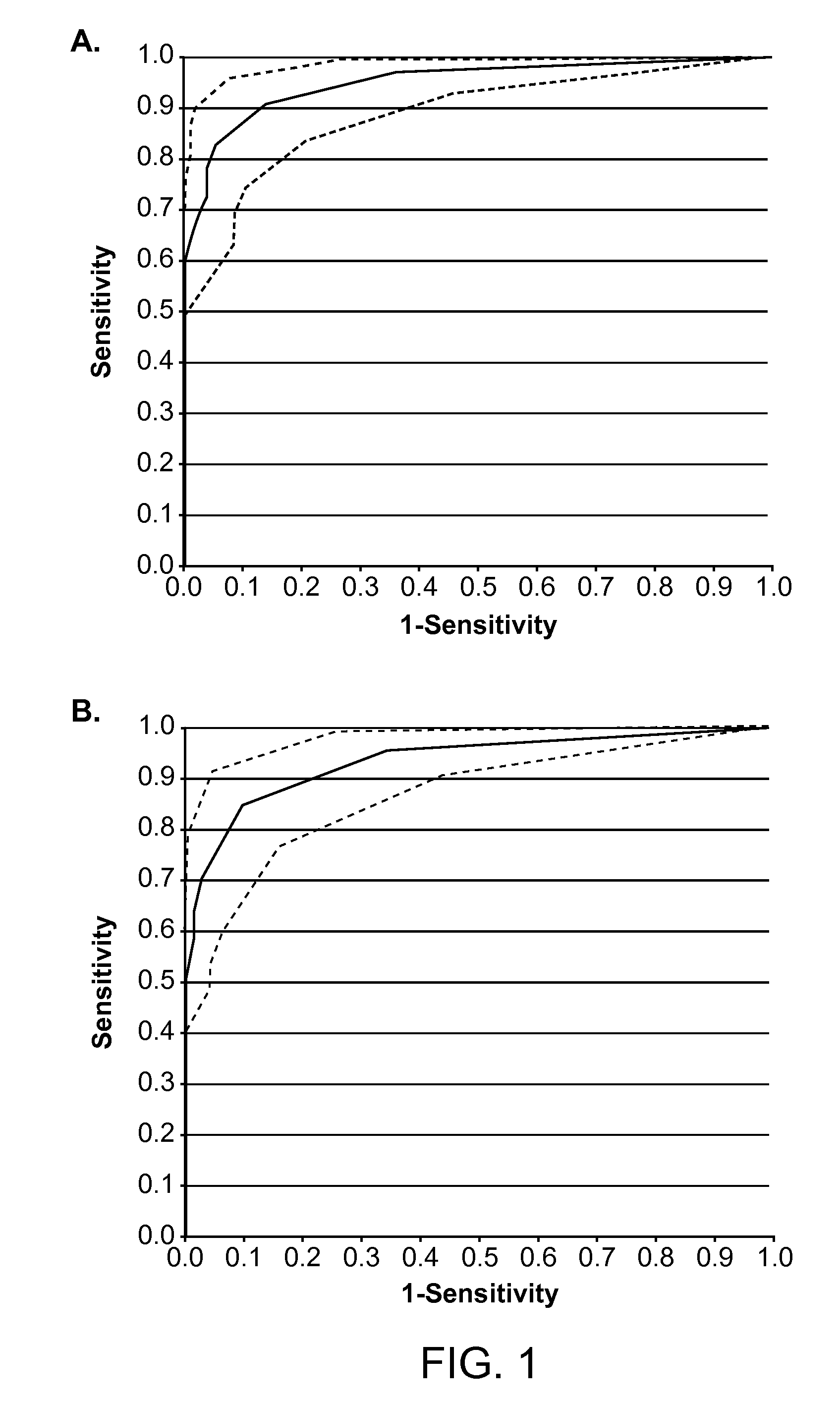
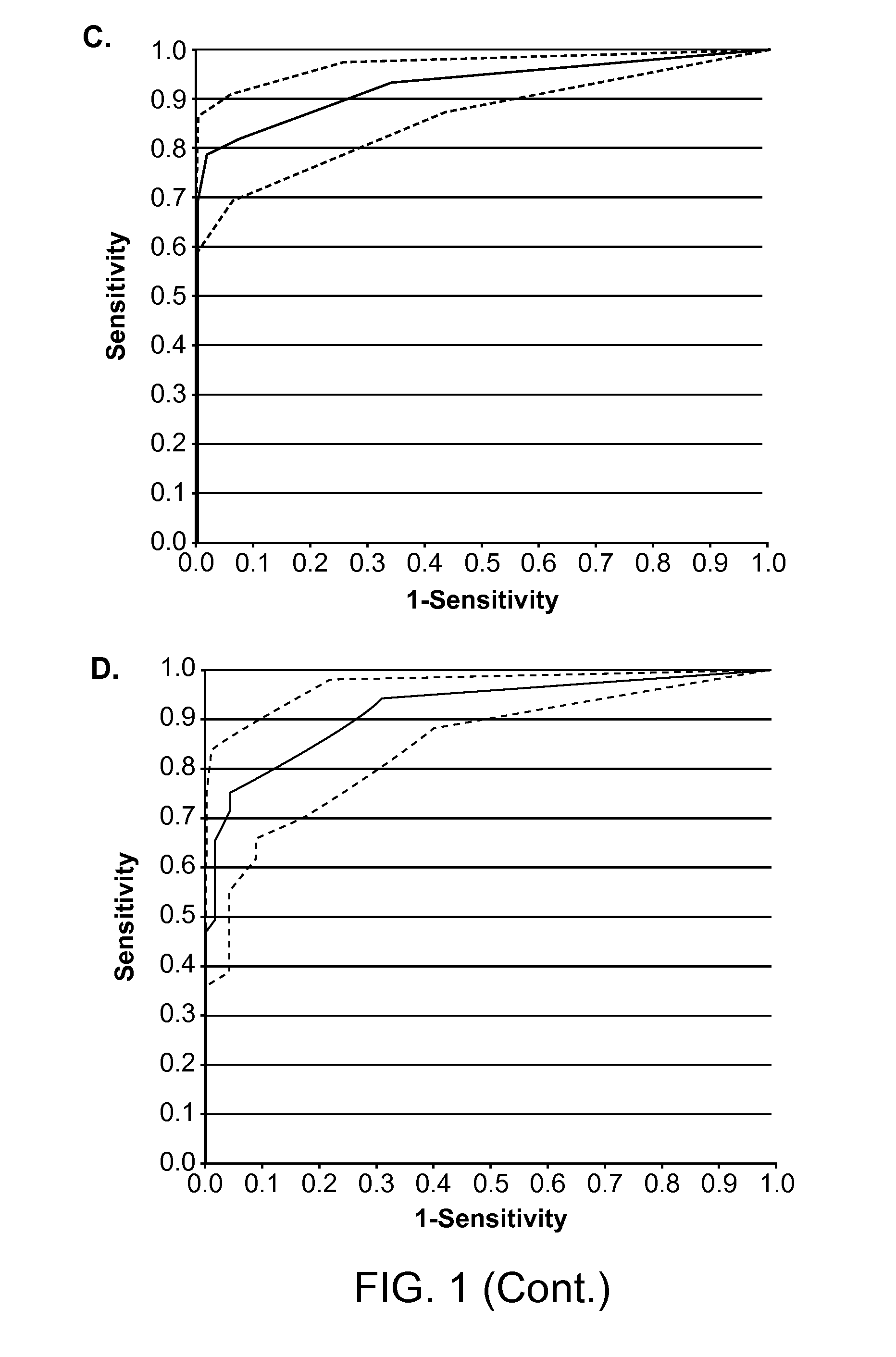
[0099]The study evaluated 68 serum samples obtained from patients with colorectal cancer and 72 serum samples from healthy controls. Patient serum samples were obtained from two different sites: Cleveland Clinic satellite facility in Florida USA (n=16) and Ryazan Central Oblast Hospital, Russia (n=47). Blood was drawn preoperatively from treatment-nave patients under local IRB approval and processed as described previously (Beck et al., Clin. Chem. 55:730-738, 2009). Normal samples were obtained from the department of Transfusion Medicine of the Georg-August University of Göttingen (n=12), the Ryazan Central Oblast Hospital (n=50), Asterand plc., Detroit, Mich., USA, (n=8), and an additional two volunteers.
Construction of Sequencing Libraries
[0100]After extraction of DNA from serum or plasma, using a standard silica-based method, a whole genome amplification was performed in duplicate. The products of the two reactions w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com