Neuroendocrine Tumors
a neuroendocrine tumor and gene expression technology, applied in the field of gene expression to classify neuroendocrine tumors, can solve the problems of unknown or uncertain tumor site origin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0116]Seventy-five (44 metastatic, 31 primary) formalin-fixed, paraffin-embedded neuroendocrine tumor samples were selected after 2-institution pathologist adjudication. The samples included subtypes gastrointestinal (n=12), pulmonary (n=22), Merkel cell (n=10), pancreatic (n=10), pheochromocytoma (n=10), and medullary thyroid carcinoma (n=11).
[0117]The following tumors were considered to have neuroendocrine differentiation: Merkel cell carcinoma, medullary thyroid carcinoma, pheochromocytoma, paraganglioma, pulmonary NEC (carcinoid, small cell carcinoma, large cell NEC), pancreatic NEC (all grades), and gastrointestinal NEC (all grades; stomach, small intestine, appendix, and colorectum). Both primary and metastatic cases were included. Excluded were some sites of “epithelial” neuroendocrine tumors (thymus, pituitary, kidney, bladder, cervix, ovary), carcinomas with occult / mixed neuroendocrine differentiation, and most of the rarer “neural” types of neuroendocr...
example 2
Classification of Neuroendocrine Tumors
[0120]Blinded samples were tested by the CancerType ID® 92-gene classifier (bioTheranostics, Inc), which makes tumor type predictions based upon quantitative PCR expression measurement for 87 gene targets and 5 reference genes. Briefly, a selected formalin fixed, paraffin embedded block was sectioned in RNase free conditions to produce one hematoxylin and eosin stained section and three unstained 7-micron sections for molecular testing. The freshly prepared slides included only a research ID. Samples were macrodissected using the H&E stained template or laser capture microdissected for tumor enrichment. Total RNA was extracted and DNase treated.
[0121]First strand cDNA was synthesized and then was pre-amplified (PreAmp, Life Technologies, Carlsbad, Calif.). Real-time PCR was then performed using an ABI 7900HT instrument quantitatively measuring the expression of 87 tumor-associated genes and 5 reference genes as previously described (Ma et al. 2...
example 3
Characteristics of Classification
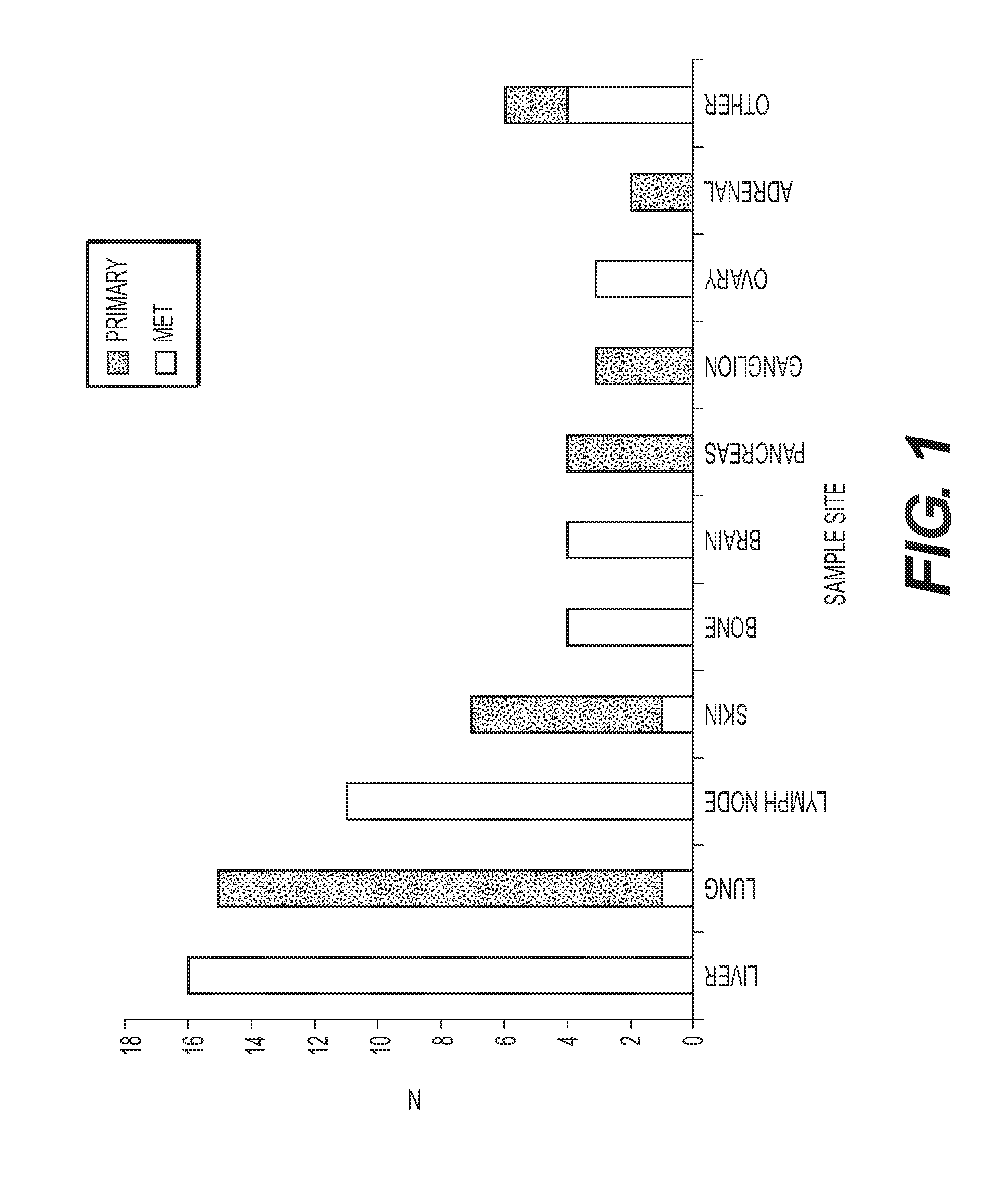
[0125]All 75 neuroendocrine tumors met quality control parameters and were classified by the assay. The cohort included 44 females and 31 males, with a mean age of 62 years (range 29 to 86). Tumor characteristics are provided in Table 4. Cases were comprised of 59% metastatic tumors and 41% primary tumors. The most common biopsy site was liver, followed by lung and lymph node (FIG. 1). The performance characteristics for the 92-gene assay predictions of neuroendocrine subtype are shown in Table 5.
TABLE 5Neuroendocrine subtypenMatchesSensSpecPPVNPVGastrointestinal12121.001.001.001.00Merkel cell10101.000.970.831.00Pancreatic1080.800.980.910.97Pheo / paraganglioma10101.001.001.001.00Pulmonary22200.911.001.000.98Thyroid Medullary11111.001.001.001.00Total75710.95
[0126]Assay sensitivities were 99% (95% CI: 0.93-0.99) for accurate classification of neuroendocrine tumors and 95% (95% CI:0.87-0.98) for identification of tumor subtype for site of origin. Positiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| CT | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com