Methods for treating pruritus
a technology of uremic pruritus and treatment methods, which is applied in the field of treatment methods of uremic pruritus, can solve the problems of difficult treatment and management of uremic pruritus, and the uremic pruritus in patients with kidney failure and/or patients undergoing dialysis can be quite disabling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 to 3
[0176]Three sustained release delivery systems were prepared by dry blending xanthan gum, locust bean gum, calcium sulfate dihydrate, and mannitol in a high speed mixed / granulator for 3 minutes. While running choppers / impellers, water was sprayed to the dry blended mixture, and granulated for another 6 minutes. Then the granulation process was stopped and the mixer / granulation bowl was scraped. While running choppers / impellers, the granulation was mixed for one more minute. After the granulation was checked for consistency, while running choppers / impellers additional water was added to the granulation and granulated for additional 3.5 minutes. The granulation was then dried to LOD (loss on drying) of less than about 4% by weight. The granulation was then milled using screen #1521-0033. The relative quantities of the ingredients are listed in Table 1.
TABLE 1Sustained Release DeliveryExample 1Example 2Example 3System Excipient%%%Xanthan Gum, NF8.012.020.0Locust Bean Gum, FCC12.018.030...
examples 4 to 7
[0177]A series of tablets containing different amounts of gum were prepared using the sustained release delivery system of Example 3. The quantities of ingredients per tablet are listed in Table 2.
TABLE 2Ex. 4Ex. 5Ex. 6Ex. 7ComponentMgMgmgmgNalbuphine60 60 60 60 HCI, USPSustained601 1201 1801 901 release deliverysystemMagnesium0.51.81.2 0.75stearate, NFTotal Weight120.5 181.8 241.2 150.75 Active:Gum1:0.51:11:1.51:0.75Tooling Size 0.2812″ 0.2812″ 0.3125″ 0.2812″Hardness (Kp)1.28.88.97.21Sustained release system of Example 3
[0178]The tablets were prepared by mixing nalbuphine with the sustained release delivery system in a mixer. The magnesium stearate was passed through a #30 mesh screen sieve and then mixed with the dry blend containing nalbuphine and the sustained release delivery system. This lubricated blend was compressed using the tooling as specified in Table 2 to make tablets of the total weight indicated.
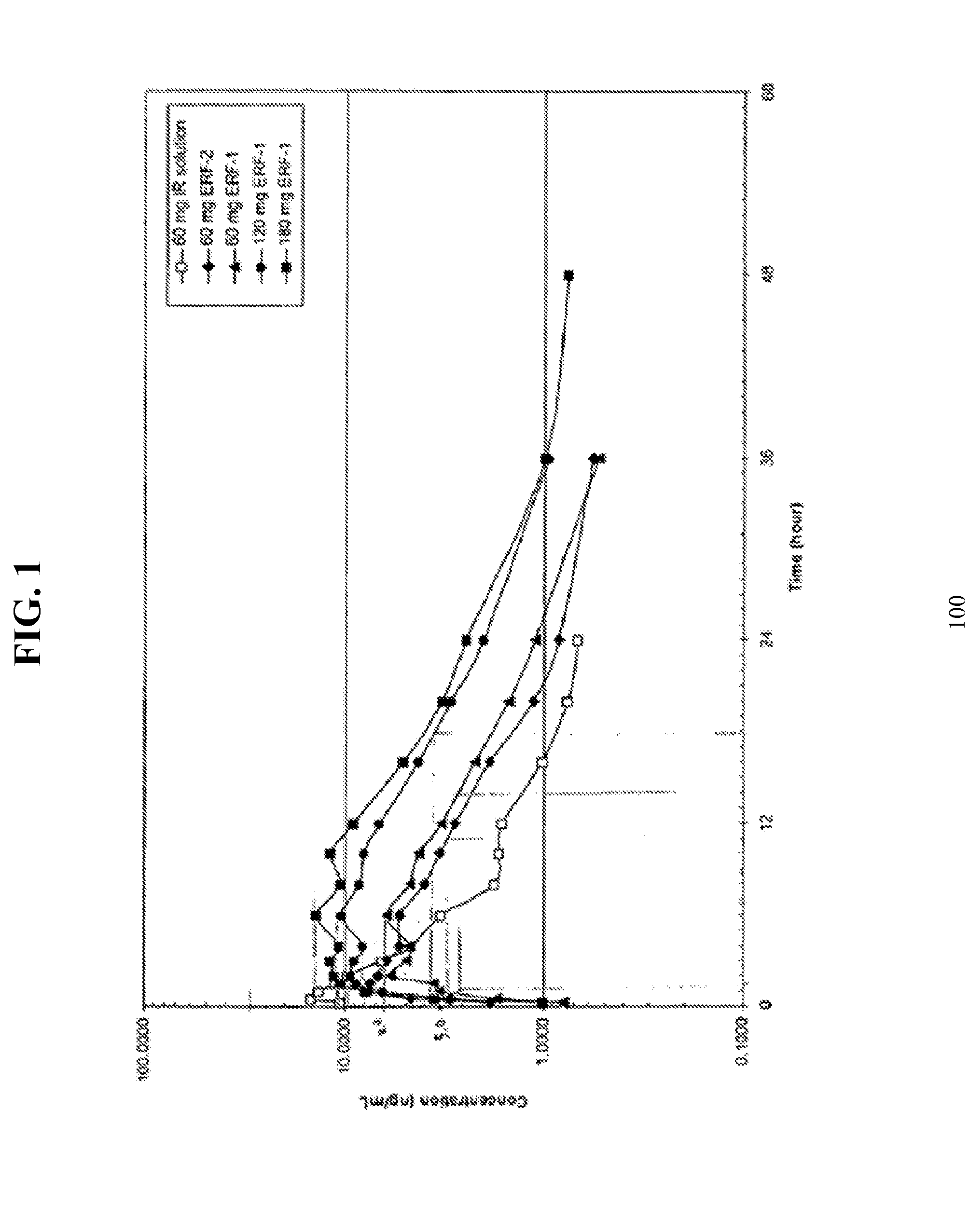
[0179]The tablets of Examples 4-7 were tested for in vitro % ...
examples 8 to 10
[0180]A series of tablets containing different amounts of gum and different sustained release delivery systems were prepared using the sustained release delivery systems of Examples 1 and 2. The quantities of ingredients per tablet are listed in Table 4.
TABLE 4Ex. 8Ex. 9Ex. 10ComponentmgMgmgNalbuphine606060HCI, USPSustained225215031003release deliverysystemMagnesium 1.43 1.1 0.8stearateTotal weight 286.4 211.1 160.8Active:Gum1:0.751:0.751:0.5Tooling Size 0.3125″ 0.3125″ 0.2812″Hardness (Kp)2017202Sustained release delivery system of Example 13Sustained release delivery system of Example 2
[0181]The tablets were prepared by first mixing nalbuphine with the sustained release delivery system in a mixer for Example 8 and in a high shear granulator for Example 9 and 10. For Examples 9 and 10, the blend was then granulated with water until consistent granulation was achieved, followed by drying in a fluidized bed dryer for 30 minutes at 70° C. The dried granules were then pass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compression pressures | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com