ANTAGONISTS OF Bcl-2 AND USES THEREOF IN INDUCTION OF APOPTOSIS
a technology of bcl-2 and induction of apoptosis, which is applied in the field of anti-bcl-2 and its use in the induction of apoptosis, to achieve the effect of better and more effective induction of apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
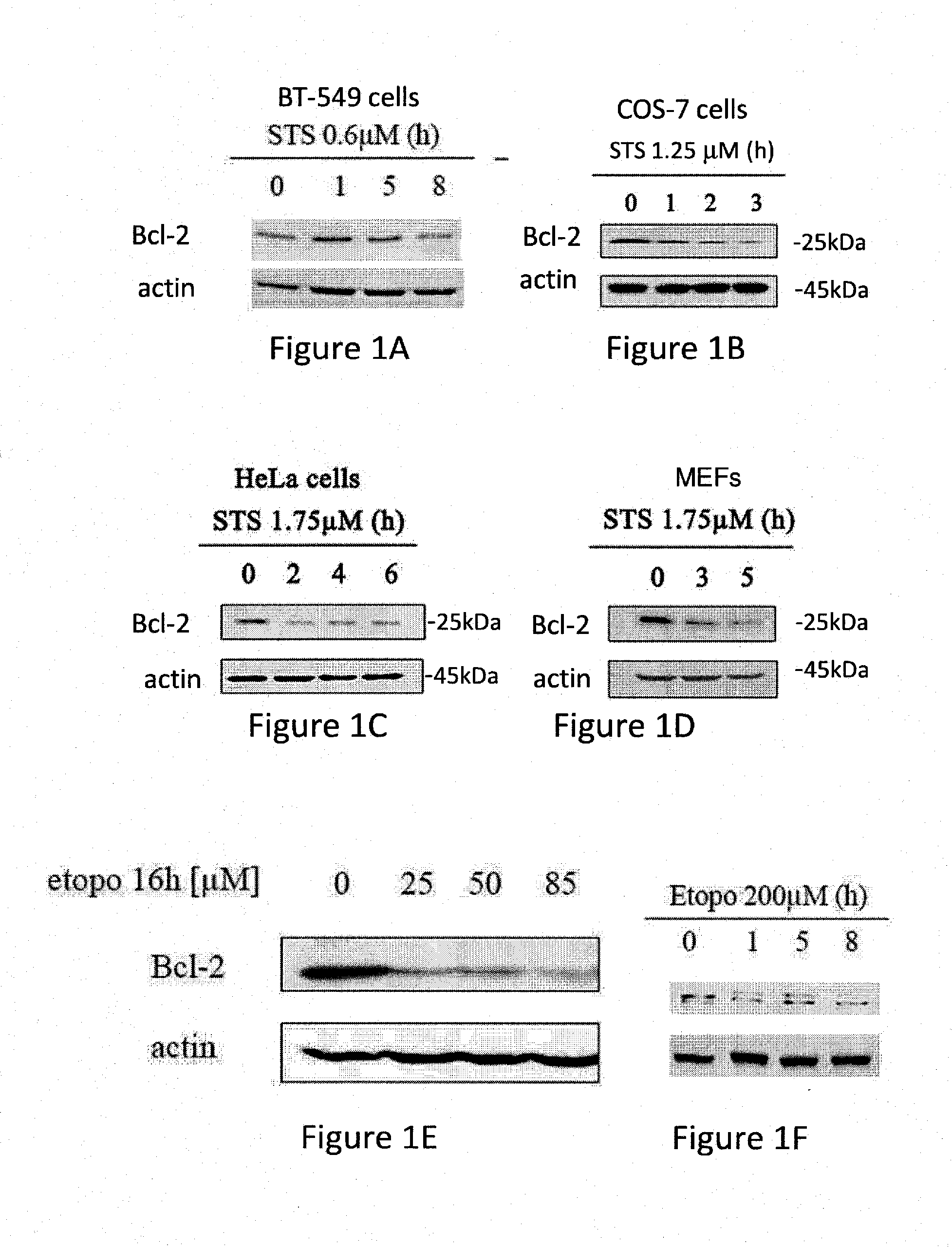
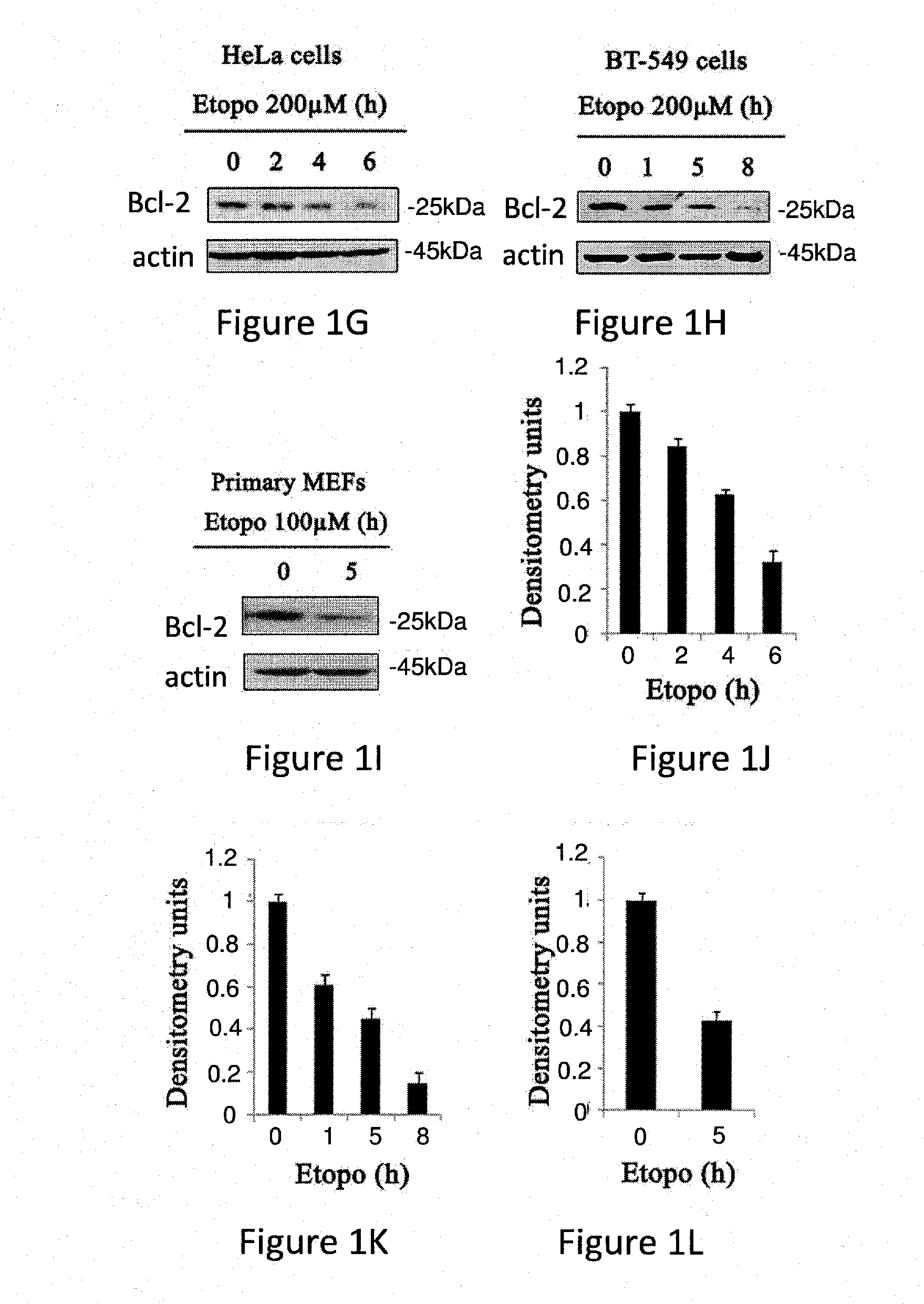
Bcl-2 Protein Levels are Down-Regulated During Apoptosis Induced by STS and Etoposide
[0340]Bcl-2 is an anti-apoptiotic factor and thus at the onset of apoptosis, the anti-apoptotic function of Bcl-2 has to be overcome. This usually occurs through interactions between Bcl-2 and the pro-apoptotic members of the Bcl-2 family (Youle and Strasser, 2008).
[0341]The role of Bcl-2 during apoptosis was studied in different cell lines and under various apoptotic conditions. The results presented in FIG. 1 show that Bcl-2 levels are down regulated upon induction of apoptosis with Staurosporine (STS) or Etoposide in different cell lines and mouse embryonic fibroblasts (MEFs).
[0342]These results are in accordance with previous data showing that Bcl-2 levels are down regulated during apoptosis induced by treatment with cisplatin, ursolic acid, Se-methylselenocystein, TNFα and ROS.
[0343]Specifically, FIGS. 1A to 1I display western blots of whole cell lysate using Bcl-2 antibodies. The levels of Bcl...
example 2
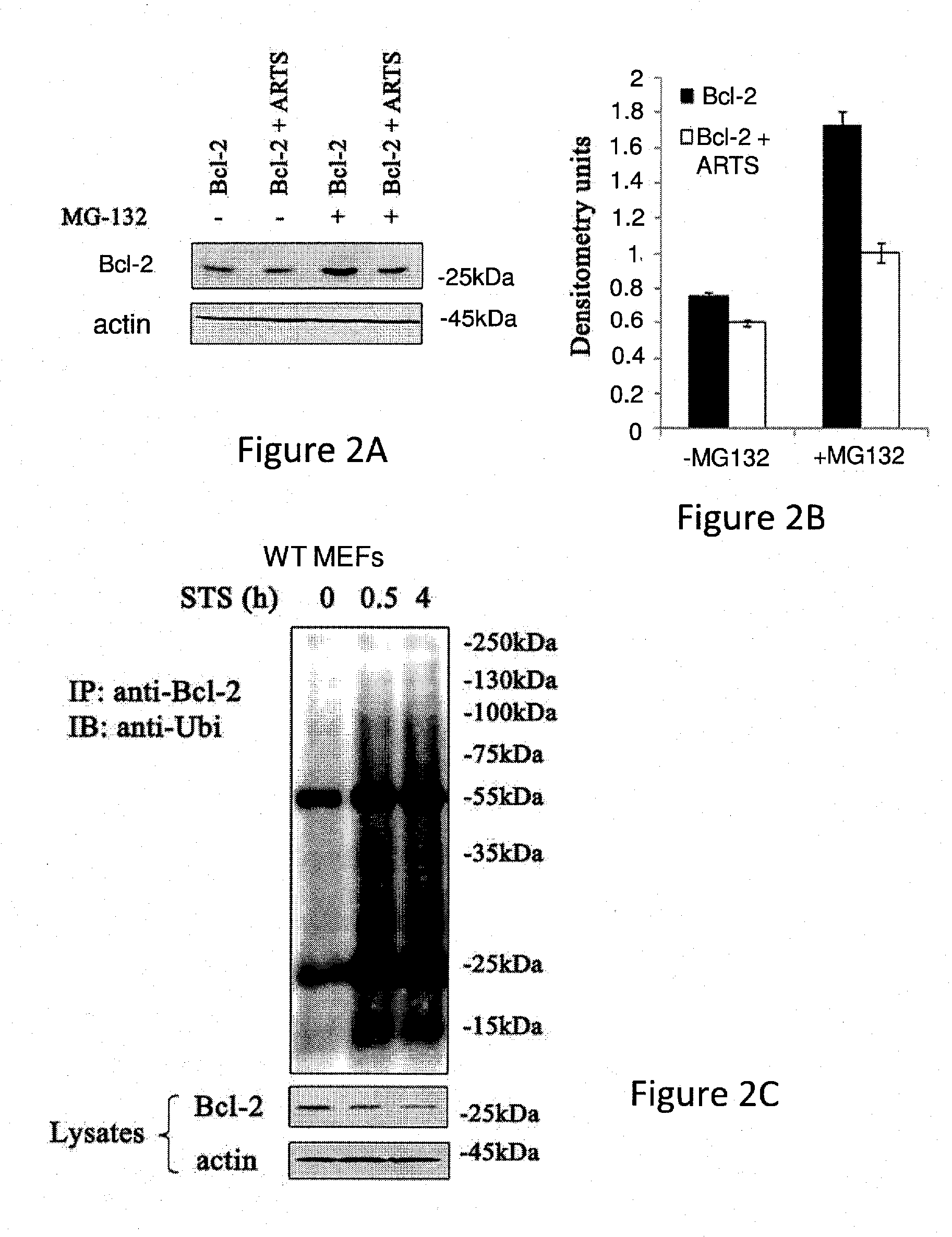
ARTS is Required for Down-Regulation of Bcl-2 Levels
[0352]Bcl-2 is known to be localized at the outer membrane of mitochondria (MOM), the endoplasmic reticulum and nuclear envelop (Kaufmann et al., 2003).
[0353]To further determine the cellular localization of Bcl-2 under apoptotic and non-apoptotic conditions, Immunofluorescence assay was conducted. HeLa cells were transfected with Bcl-2 and the cells were treated with the apoptotic inducer Staurosporine (STS) for 60 and 180 minutes.
[0354]Cells in which Bcl-2 is detected at mitochondria (co-localize with MitoTracker), or at the cytosol (showing diffused pattern of staining) were counted. FIGS. 3A to 3C show that at time 0, all Bcl-2 was localized to the mitochondria. Sixty minutes following STS treatment, 63% of cells exhibited Bcl-2 in their cytosol, and following 180 minutes of STS treatment, most of the cells (94%) exhibited Bcl-2 in the cytosol.
[0355]It has been recently shown by some of the inventors that similarly to Bcl-2, AR...
example 3
Bcl-2, ARTS and XIAP Form a Complex
[0362]The mechanism by which ARTS regulates Bcl-2 levels was further tested using a pull-down assay in COS-7 cells which were co-transfected with Bcl-2 and ARTS expression vectors. Pull-down assays were performed using agarose anti-myc beads followed by Western blot analysis using mouse anti-ARTS and anti-Bcl-2 antibodies.
[0363]The results presented in FIG. 4A show that ARTS forms a complex with Bcl-2. Interestingly, the complex is degraded upon induction of apoptosis with Etopo.
[0364]It has been previously shown by part of the inventors that ARTS binds directly to XIAP and functions as antagonist to XIAP both in vitro and in vivo (Bornstein et al., 2011; Edison et al., 2012a; Garcia-Fernandez et al., 2010; Garrison et al., 2010; Gottfried et al., 2004). XIAP is an E3-ligase and this activity is essential for its anti-apoptotic activity; Schile et al., 2008).
[0365]Because both ARTS and Bcl-2 are localized at the MOM, and since ARTS binds to XIAP, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com