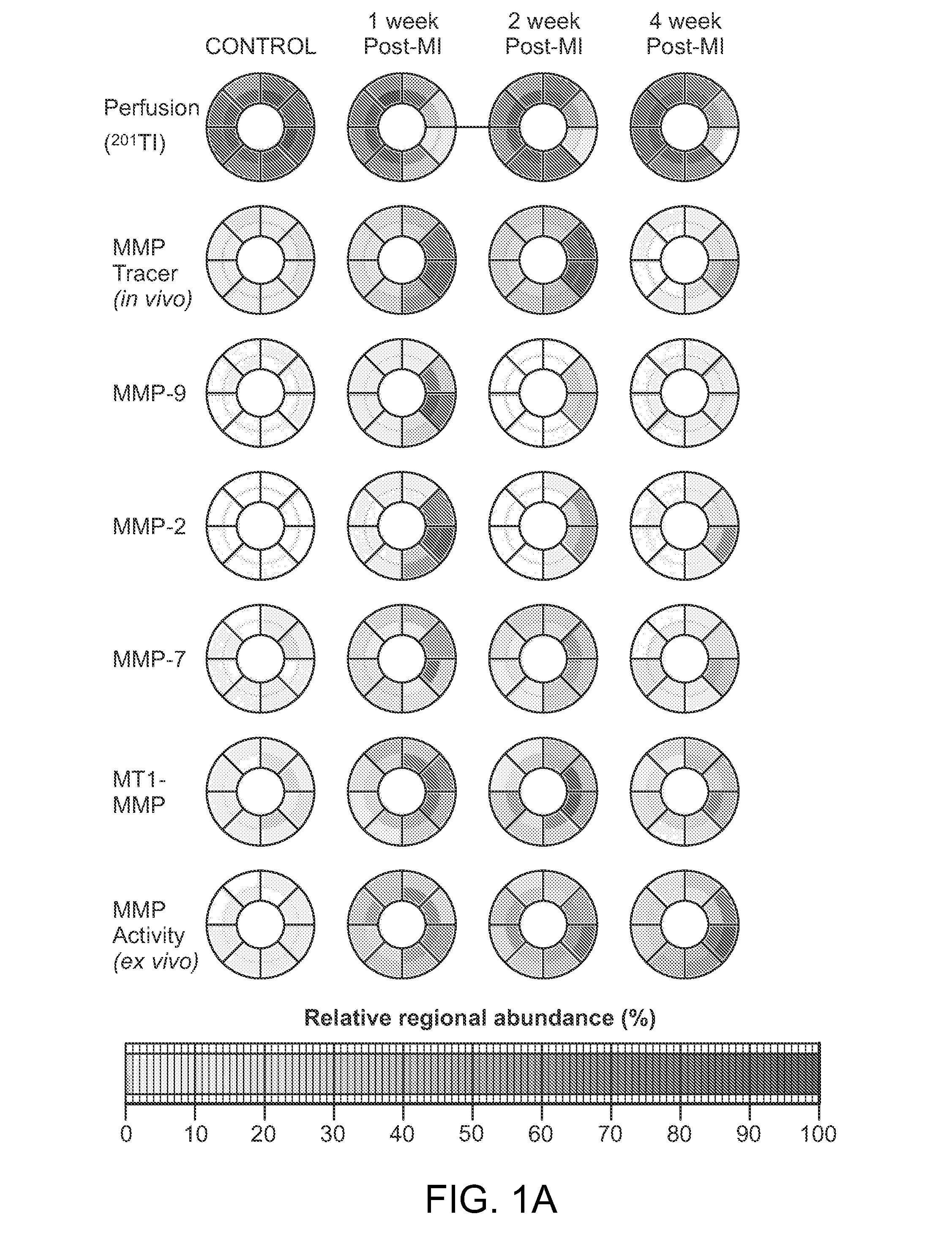
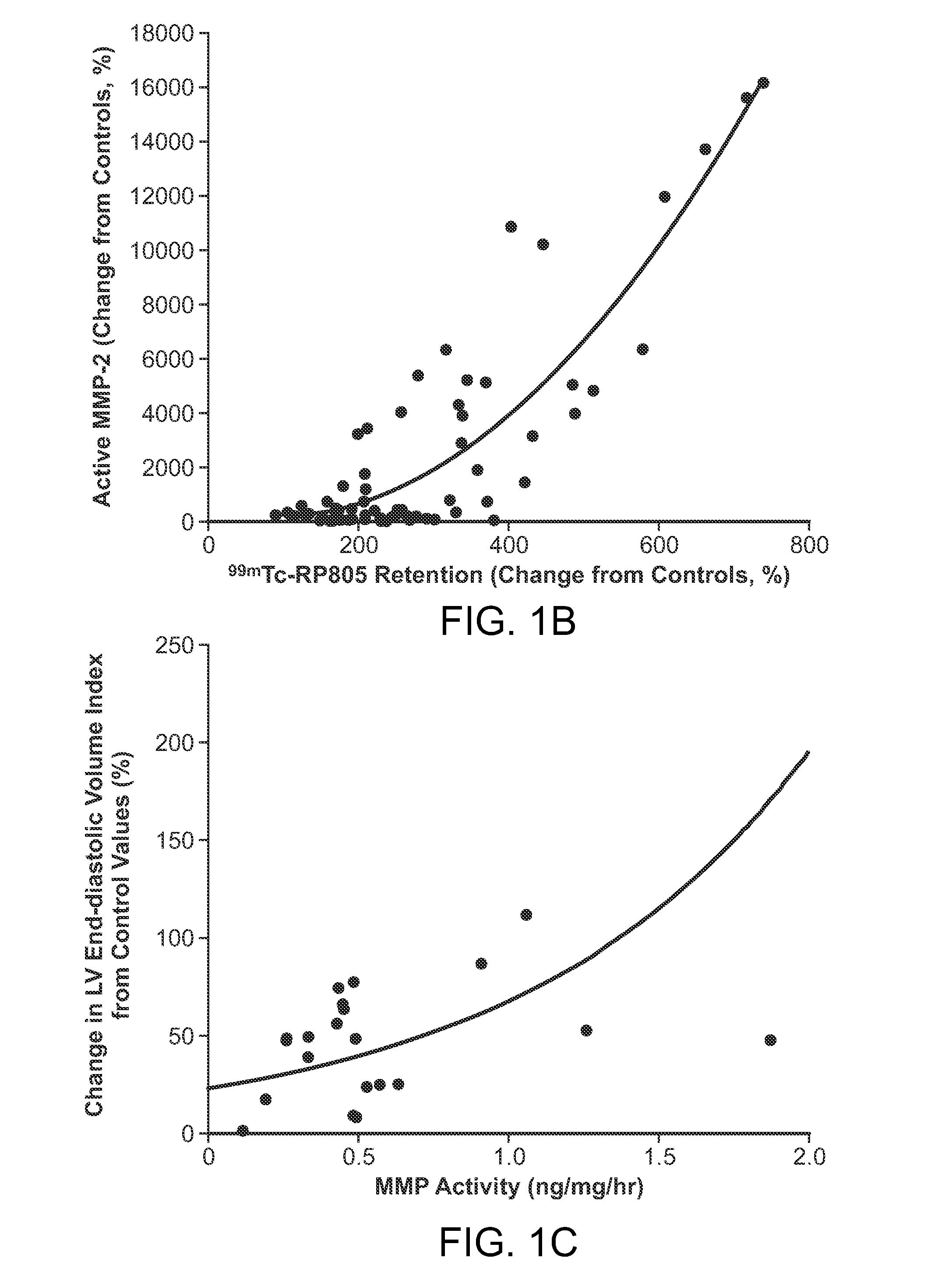
Evaluation of presence of and vulnerability to atrial fibrillation and other indications using matrix metalloproteinase-based imaging
a technology of matrix metalloproteinase and atrial fibrillation, which is applied in the field of evaluating the presence and vulnerability of atrial fibrillation and other indications using matrix metalloproteinase-based imaging, can solve the problems of correlated with increased risk of af and cavd, and achieve the effect of increasing the uptake of imaging agents and increasing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
(1) In some embodiments, the compound is of embodiment 1 of this first non-limiting set of embodiments, wherein the compound comprises:
a) 1-10 targeting moieties
b) a chelator (Ch); and
c) 0-1 linking groups (Ln) between the targeting moiety and chelator;
wherein the targeting moiety is a matrix metalloproteinase inhibitor; and
wherein the chelator is capable of conjugating to a cytotoxic radioisotope.
(2) A compound according to embodiment 1, wherein the targeting moiety is a matrix metalloproteinase inhibitor having an Inhibitory constant Ki of <1000 nM.
(3) A compound according to embodiment 1, wherein the targeting moiety is a matrix, metalloproteinase inhibitor having an inhibitory constant Ki of <100 nM.
(4) A compound according to any one of embodiments 1-3, comprising 1-5 targeting moieties.
(5) A compound according to embodiment 1, comprising one targeting moiety.
(6) A compound according to any one of embodiments 1-5, wherein the targeting moiety is a matrix metalloproteinase inhib...
embodiment 47
(51) In some embodiments, a radiopharmaceutical wherein the cytotoxic radioisotope is selected from the group consisting of beta particle emitters, alpha particle emitters, and Auger electron emitters.
(52) In some embodiments, a radiopharmaceutical according to embodiment 47 wherein the cytotoxic radioisotope is selected from the group consisting of: 186Re, 188Re, 153Sm, 166Ho, 177Lu, 149Pm, 90Y, 212Bi, 103Pd, 109Pd, 159Gd, 140La, 198Au, 199Au, 169Yb, .sup.175Yb, 165Dy, 166Dy, 67Cu, 105Rh, 111Ag, and 192Ir.
(53) In some embodiments, a radiopharmaceutical according to embodiment 47 wherein the cytotoxic radioisotope is selected from the group consisting of: 186Re, 188Re, 153Sm, 166Ho, 177Lu, 149Pm, 90Y, 212Bi, 103Pd, 105Rh,
(54) In some embodiments, a radiopharmaceutical according to embodiment 47 wherein the cytotoxic radioisotope is selected from the group consisting of: 186Re, 188Re, 153Sm, 166Ho, 177Lu, 149Pm, 90Y, and 212Bi.
(55) In some embodiments, a composition comprising a com...
embodiment 36
(41) A diagnostic agent wherein the paramagnetic metal ion is selected from the group consisting of Gd(III), Dy(III), Fe(III), and Mn(II).
(42). A diagnostic agent according to embodiment 36 wherein the x-ray absorber is a metal is selected from the group consisting of: Re, Sm, Ho, Lu, Pm, Y, Bi, Pd, Gd, La, Au, Au, Yb, Dy, Cu, Rh, Ag, and Ir.
(43) A diagnostic composition comprising a compound according to any one of embodiments 1-42 or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier.
(44) A kit comprising a compound of to any one of embodiments 1-42, or a pharmaceutically acceptable salt form thereof and a pharmaceutically acceptable carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| real time RT- | aaaaa | aaaaa |
| real time RT- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com