1,2,4-Oxadiazole Derivatives as Immunomodulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
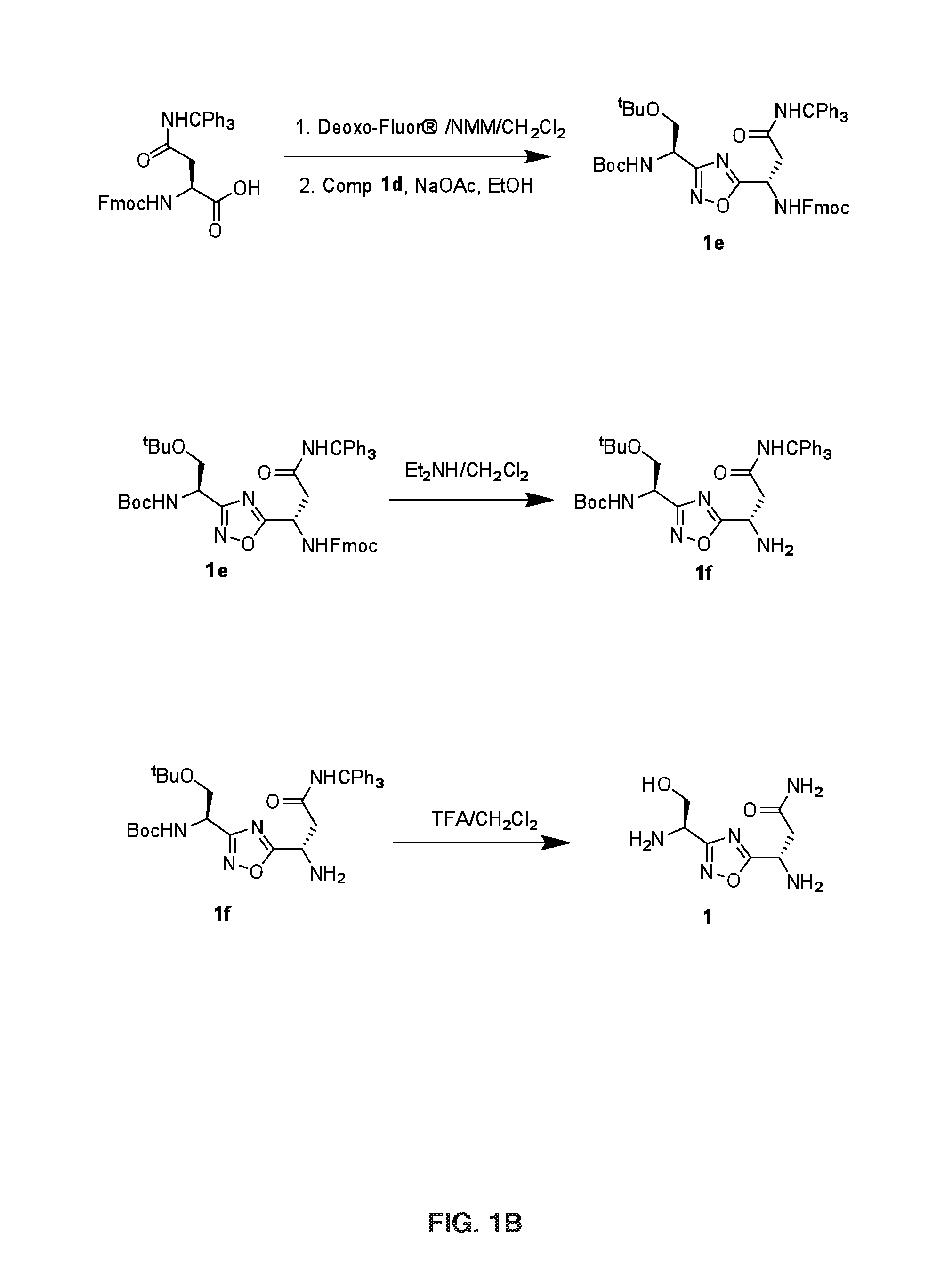
Synthesis of Compound 1
[0128]
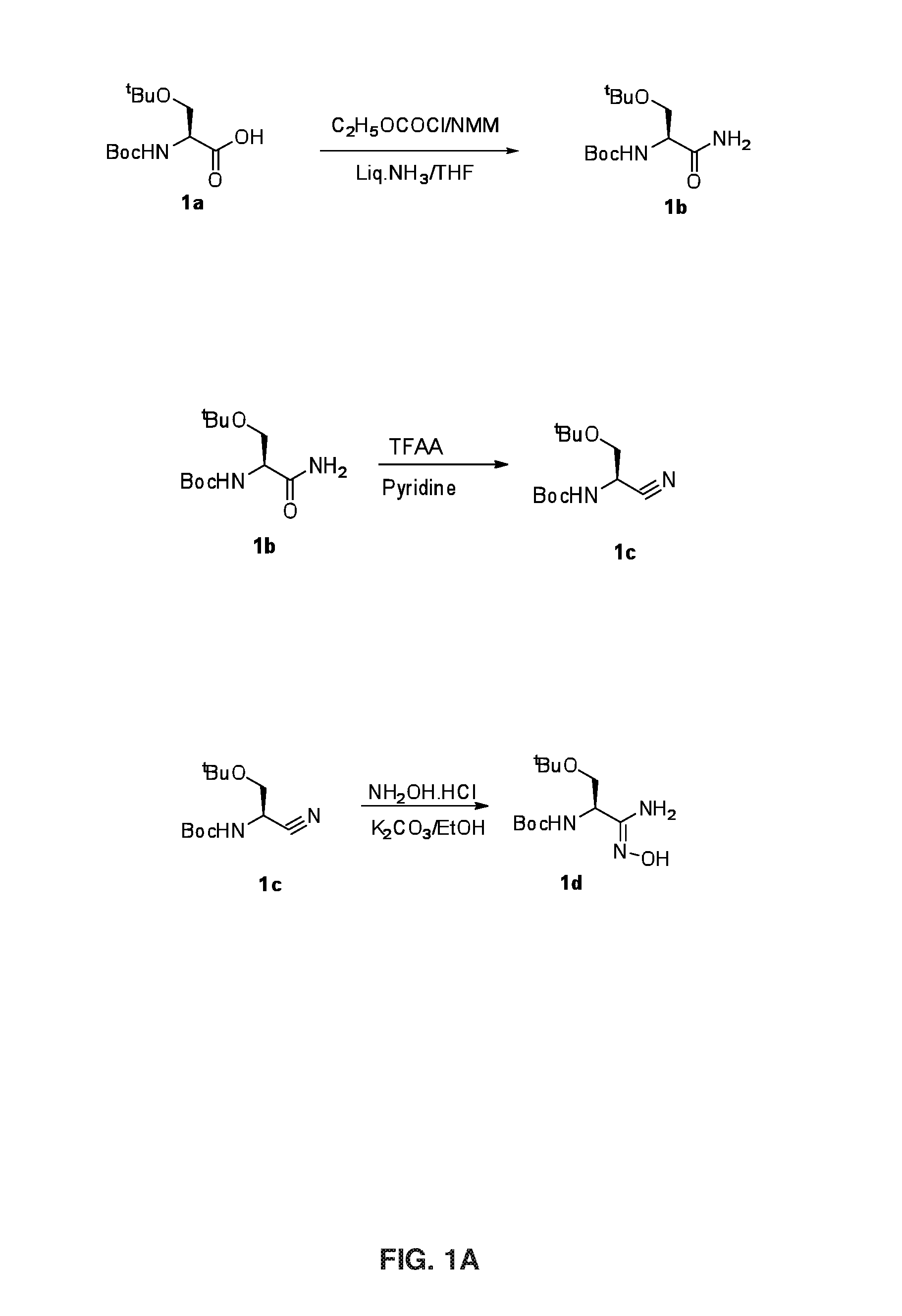
[0129]FIG. 1A illustrates Steps 1a, 1b and 1c.
[0130]Step 1a: Ethylchloroformate (1.5 g, 13.78 mmol) and N-Methylmorpholine (1.4 g, 13.78 mmol) were added to a solution of compound 1a (3 g, 11.48 mmol) in THF (30 mL) and stirred at −20° C. After 20 min. liquid ammonia (0.77 g, 45.92 mmol) was added to the active mixed anhydride formed in-situ and stirred at 0-5° C. for 20 min. The completeness of the reaction was confirmed by TLC analysis. The reaction mixture was evaporated under reduced pressure and partitioned between water and ethyl acetate. Organic layer was washed with NaHCO3, citric acid, brine solution, dried over Na2SO4 and evaporated under reduced pressure to get 2.9 g of compound 1b (Yield: 96.3%). LCMS: 261.0 (M+H)+.
[0131]Step 1 b: Trifluroacetic anhydride (9.7 g, 46.0 mmol) was added to a solution of compound 1b (8 g, 30.7 mmol) in pyridine (24.3 g, 307.0 mmol) and stirred at room temperature for 3 h. The completeness of the reaction was conf...
example 2
Synthesis of Compound 2
[0136]
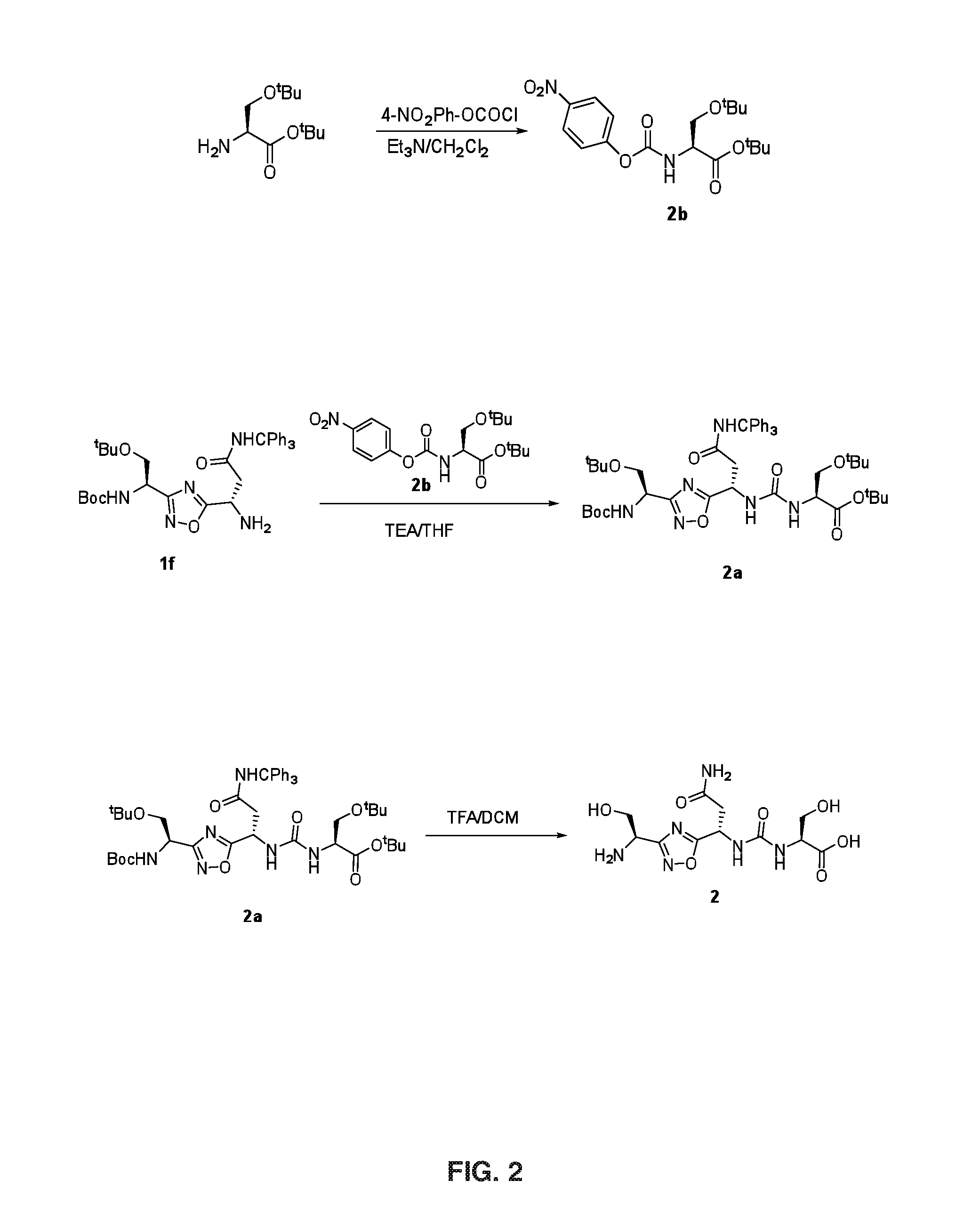
[0137]FIG. 2 illustrates Steps 2a and 2b.
[0138]Step 2a: The urea linkage was carried out by the coupling compound 1f (2.7 g, 4.39 mmol) in THF (30 mL) at room temperature with compound 2b (1.67 g, 4.39 mmol). The coupling was initiated by the addition of TEA (0.9 g, 8.78 mmol) in THF (10 m L) and the resultant mixture was stirred at room temperature. After completion of 20 h, THF was evaporated from the reaction mass, and partitioned between water and ethyl acetate. Organic layer was washed with water, brine, dried over Na2SO4 and evaporated under reduced pressure to get compound 2a, which was further purified by silica gel column chromatography (Eluent: 0-50% ethyl acetate in hexane) to afford 3.46 g of compound 2a (Yield: 92.10%). LCMS 857.4 (M+H)+.
[0139]Step 2b: To a solution of compound 2a (0.22 g, 0.25 mmol) in CH2Cl2 (5 m L), trifluoroacetic acid (5 mL) and catalytic amount of triisopropylsilane were added and stirred for 3 h at room temperature. T...
example 3
Synthesis of Compound 3
[0141]
[0142]The compound was synthesised using similar procedure as depicted in Example 1 (compound 1) and D-amino acids are linked up in reverse order. Boc-D-Thr(tBu)-OH was used in place of Boc-Ser(tBu)-OH (compound 1a, Example 1) and Fmoc-D-Asn(trt)-OH in place of Fmoc-Asn(trt)-OH to yield 0.15 g crude material of the title compound 3. LCMS: 230.1 (M+H)+.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com