Gender, viability and/or developmental stage determination of avian embryos in ovo
a technology of avian embryos and developmental stages, which is applied in the field of determining the gender, developmental stage and/or viability of an avian embryo in ovo, can solve the problems of reducing the capacity of hatchery incubators, culling billions of male chicks every year, and not being able to reliably determine the gender and other characteristics of unhatched birds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protocol Metabolic Profiling in Ovo
[0062]Gallus gallus domesticus eggs were incubated at 37.8° C., turning every hour, in an incubator model 50, commercially obtainable from MS Broedmachines V.O.F.
[0063]One group of 12 eggs was incubated for 9 days, a second group of 12 eggs for 10 days, and a third group of 12 eggs for 11 days.
[0064]Eggs were taken out of the incubator, placed in a paper holder, under a microscope, with the air sack up. The shell and membranes were punctured and broken open around the air sack, leaving the inner membranes intact. Using light, the blood vessels running over the inner shell membrane were located, and a small puncture avoiding the blood vessels was made through the inner and outer membranes into the allantoic cavity.
[0065]The egg was skewed and a 1 ml pipette was then used to blow air into the cavity, after which 1.5 to 2 ml of allantoic fluid was extracted using the pipette. This was transferred into a cryovial, which was immediately plunged into liq...
example 2
Protocol Metabolic Profiling in Ovo
[0069]Gallus gallus domesticus eggs were incubated at 37.8° C. at a commercial hatchery, turning every hour, in an industrial incubator commercially available from Petersime Nev. One group of 50 eggs was incubated for 8 days, a second group of 50 eggs for 9 days, and a third group of 50 eggs for 10 days.
[0070]Eggs were taken out of the incubator, placed in a plastic holder, under a microscope, with the air sack up.
[0071]The shell and membranes were punctured and broken open around the air sack, leaving the inner membranes intact. Using light, the blood vessels running over the inner shell membrane were located, and a small puncture avoiding the blood vessels was made through the inner and outer membranes into the allantoic cavity.
[0072]The egg was skewed and a 1 ml pipette was then used to blow air into the cavity, after which 1.5 to 2 ml of allantoic fluid was extracted using the pipette. This was transferred into a cryovial, which was immediately...
example 3
Determination of Embryo Age
[0084]Example 1 was repeated, however analysing the following developmental markers that were found relevant for the determination of the developmental stage, or age of the embryos.
TABLE 2Measured Data for Days 9 to 119 days (μM / mL,DevelopmentStandard deviation in10 days11 daysMarkerbrackets))(μM / mL)(μM / mL)Trimethylglycine0.75 (0.27)0.65 (0.2) 0.43 (0.13)(Betaine)Aspartate and9.35 (3.2) 7.72 (2.75)5.34 (3.91)AsparagineGlutamate and41.5 (7.71)37.1 (3.94)31.7 (5.33)GlutamineProline12.7 (2.69) 11 (1.58)9.02 (1.59)
[0085]The above data clearly indicates that the developmental stage of en embryo can be determined from one or more developmental markers.
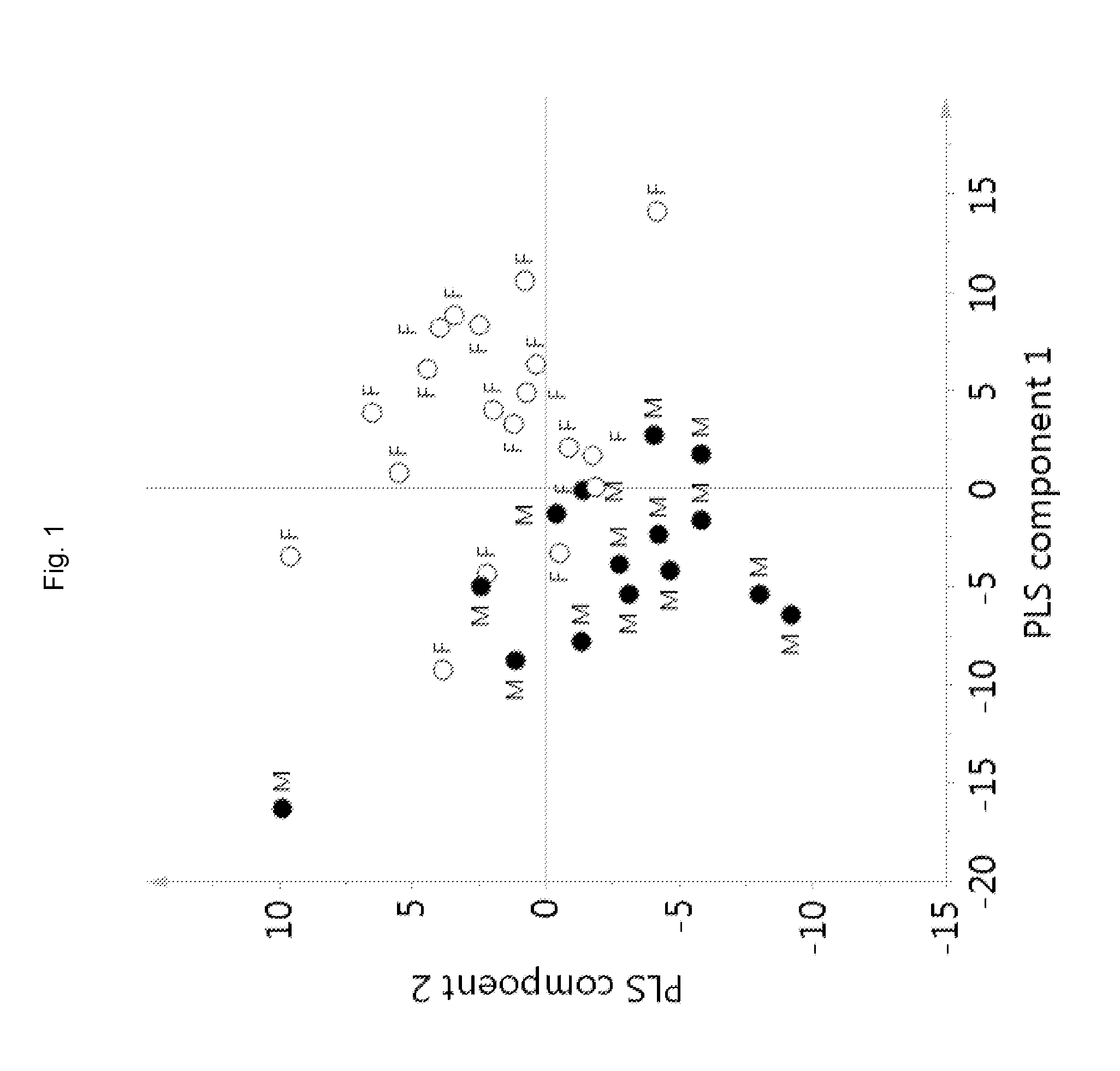
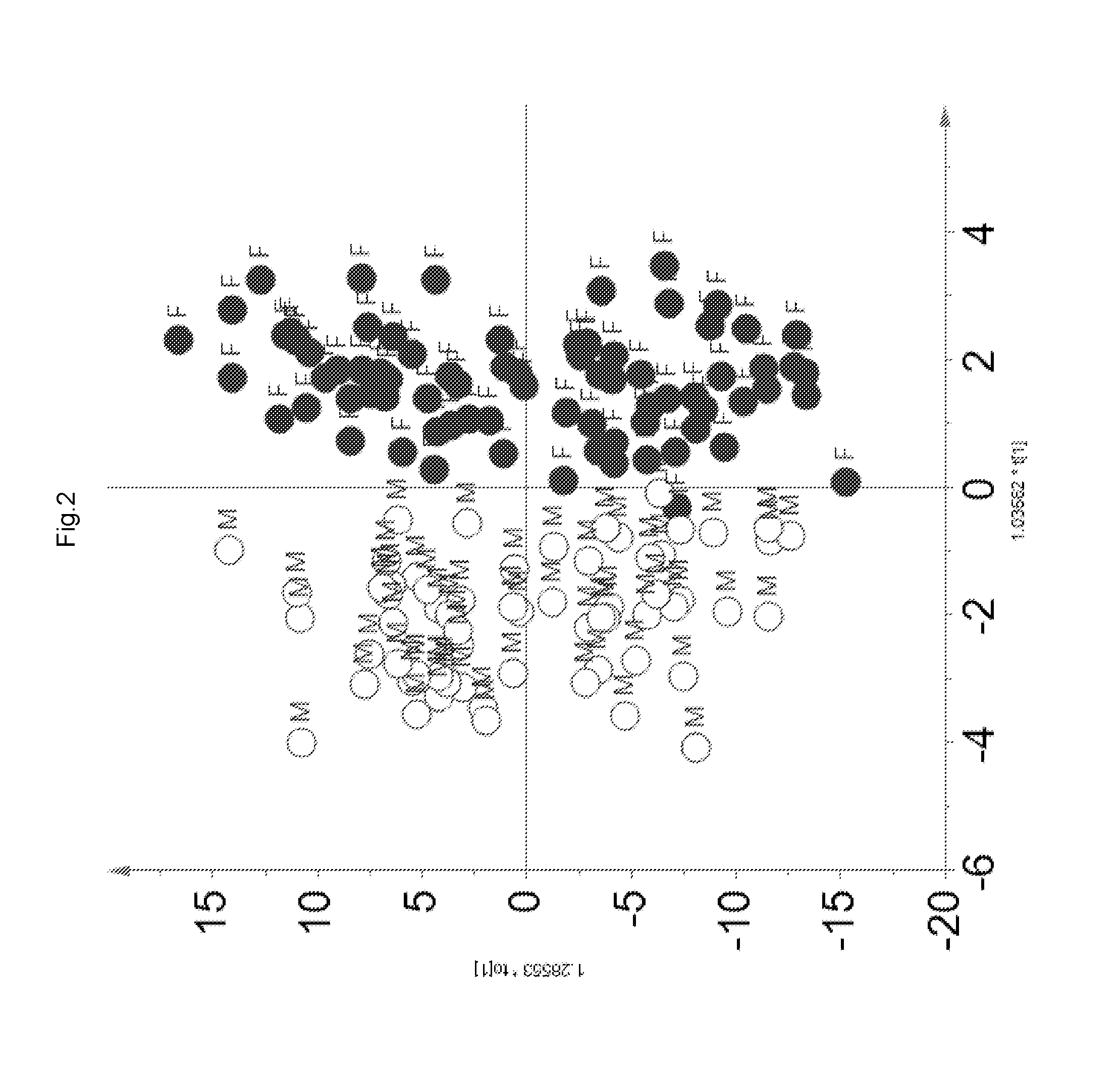
[0086]A partial least square modelling, an unsupervised multivariate data analysis, was employed for the 1H NMR data to group samples based on all the metabolites detected in 1H NMR. The most important information obtained was the correlation between two data sets, i.e. the measured 1H NMR signals (metabolites) and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com