catalyst
a technology of bisindenyl and catalyst, which is applied in the field of new bisindenyl catalysts, can solve the problems of low melting of homopolymer, polypropylene manufacturing, and polypropylene manufacturing limitations,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
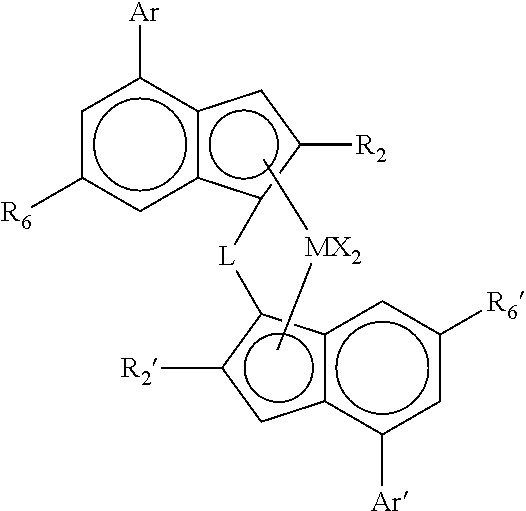
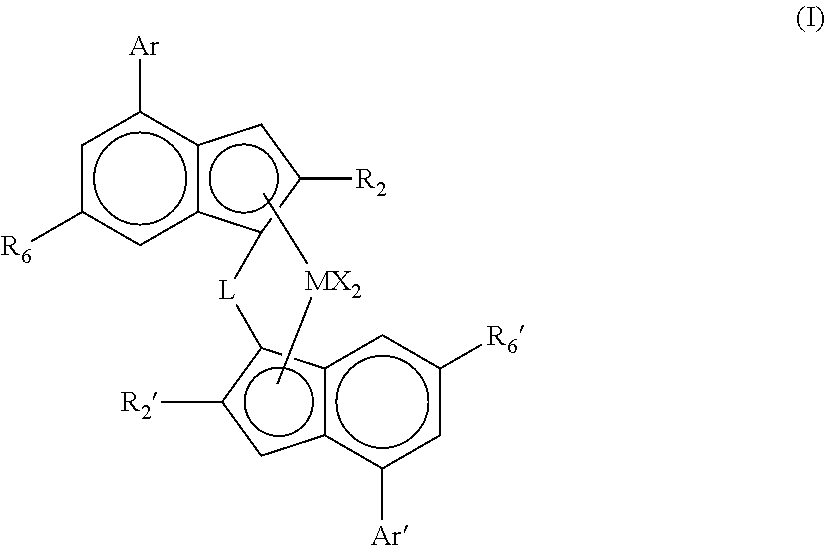
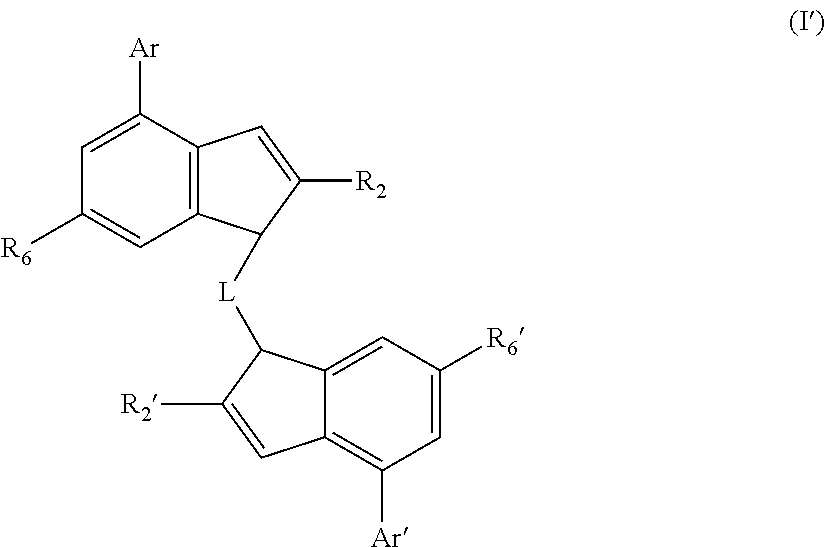
[0063]The catalysts of the invention preferably comprise a complex of formula (I)″
[0064]wherein
[0065]M is zirconium or hafnium;
[0066]each X is a sigma ligand;
[0067]L is a divalent bridge selected from —R′2C—, —R′2C—CR′2—, —R′2Si—, —R′2Si—SiR′2—, —R′2Ge—, wherein each R′ is independently a hydrogen atom, C1-20-alkyl, tri(C1-20-alkyl)silyl, C6-20-aryl, C7-20-arylalkyl or C7-20-alkylaryl;
[0068]R2 is a C1-20-hydrocarbyl radical;
[0069]R2′ is a C1-20-hydrocarbyl radical;
[0070]R6 is a linear or branched aliphatic C1-20-hydrocarbyl group, SR9 or OR9;
[0071]R6′ is a linear or branched aliphatic C1-20-hydrocarbyl group, SR9′ or OR9′;
[0072]with the proviso that neither R6 or R6′ represents a group having a quaternary carbon atom attached directly to the indenyl ring;
[0073]R9 is a C1-20-hydrocarbyl group;
[0074]R9′ is a C1-20-hydrocarbyl group;
[0075]Ar is a C6-12-aryl or C5-12-heteroaryl group optionally carrying one or more substituents R8;
[0076]Ar′ is a C6-12-aryl or C5-12-heteroaryl group opti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com