Anti-tweakr antibodies and uses thereof
a technology of anti-tweakr and antibodies, which is applied in the field of anti-tweakr antibodies, can solve the problems of lack of species cross-reactivity, lack of e.g., and less clear signaling pathways responsible for cell killing via tweakr, and achieve the effect of increasing efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody Generation from Dyax Antibody Library
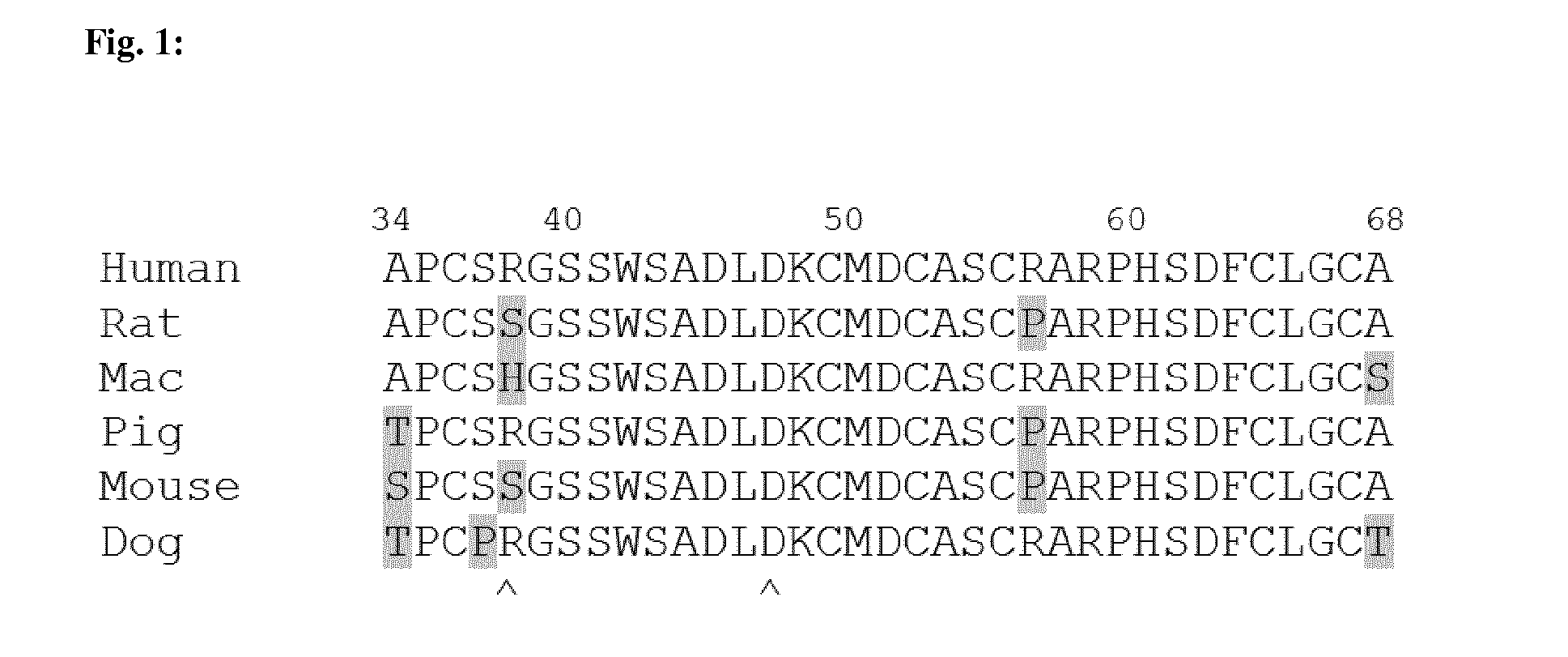
[0406]A fully human antibody phage display library (Hoet R M et al, Nat Biotechnol 2005; 23(3):344-8) was used to isolate TWEAKR-specific, human monoclonal antibodies of the present invention by protein panning (Hoogenboom H. R., Nat Biotechnol 2005; 23(3):1105-16) with dimeric Fc-fused extracellular domains of human and murine TWEAKR as immobilized target.
TABLE 1List of recombinant antigens used for antibody selectionNomenclatureDescriptionSEQ ID NOTPP-599HUMAN-TNFRSF12Aaa28-80-hIgG1-Fc138TPP-601MURINE-TNFRSF12Aaa28-80-hIgG1-Fc137
[0407]The antigens were biotinylated using an approximately 2-fold molar excess of biotin-LC-NHS (Pierce; Cat. No. 21347) according to manufacturer's instructions and desalted using Zeba desalting columns (Pierce; Cat. No. 89889). Washed Magnetic beads (Dynabeads) were incubated o / n with 200 nM of biotinylated human antigen at 4° C. and blocked for 1 h at 4° C. with blocking buffer (PBS with 3% BSA, 0.05% Tween...
example 2
Biochemical Characteristics of the Antibody
Determination of Binding Affinities by Biacore Analysis:
[0414]Binding affinities of anti-TWEAKR antibodies were determined by surface plasmon resonance analysis on a Biacore T100 instrument (GE Healthcare Biacore, Inc.). Antibodies were immobilized onto a CM5 sensor chip through an indirect capturing reagent, anti-human IgG(Fc). Reagents from the “Human Antibody Capture Kit” (BR-1008-39, GE Healthcare Biacore, Inc.) were used as described by the manufacturer. Anti-TWEAKR antibodies were injected at a concentration of 10 μg / ml at 10 μl / min for 10 sec.
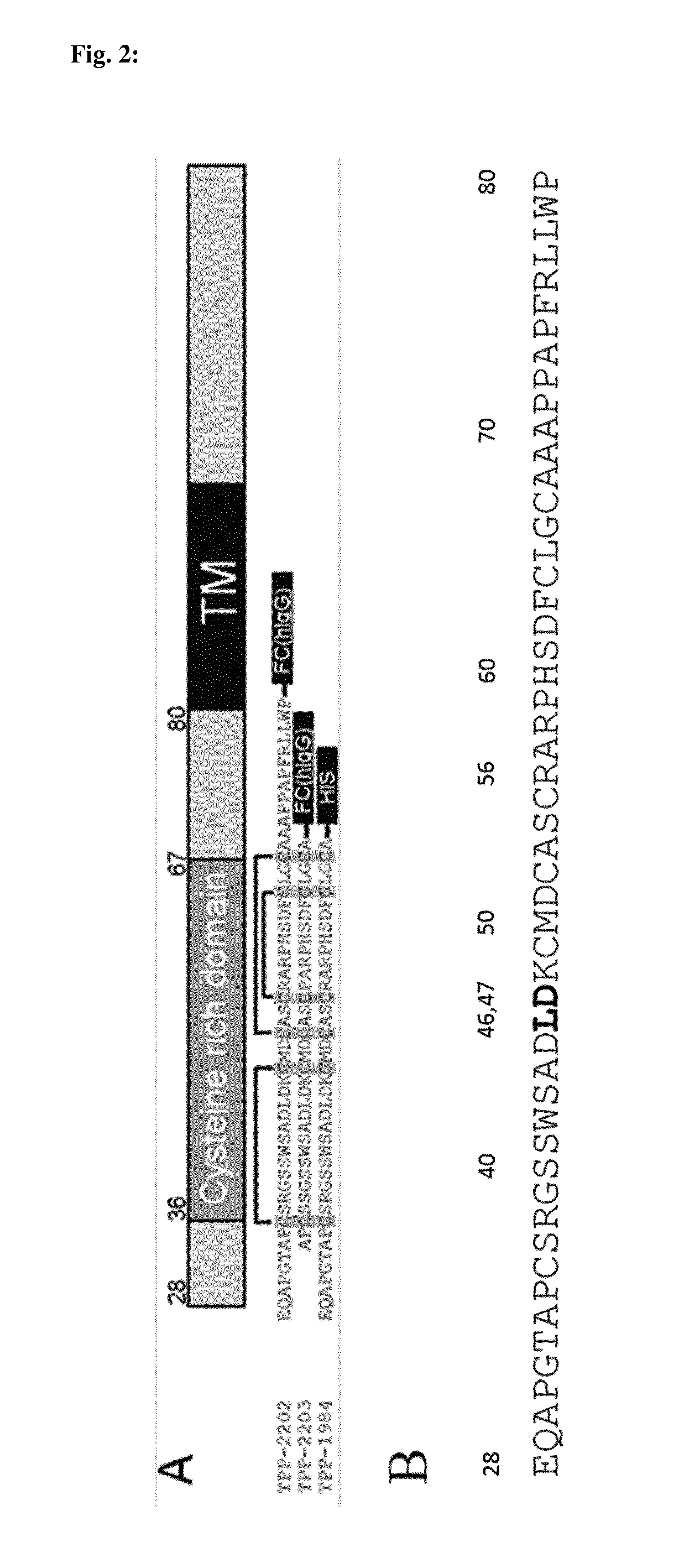
TABLE 3List of recombinant antigen (TWEAKR) used for affinity measurementCat. No.(FitzgeraldSEQNomenclatureDescriptionOriginInc)ID NOTPP-2305hTNFRSF12Aaa28-80Human30R-AT080168
TABLE 4List of antibodies used for affinity measurementsSEQ ID NONomenclatureDescriptionLight chainHeavy chainP3G5 (TPP-Murine IgG2a1211222195)P4A8 (TPP-Human IgG11251261324)P2D3 (TPP-Murine IgG2a1311322196)136.1 (TPP-Murin...
example 3
Binding of Anti-TWEAKR Antibodies to Cell Surface of Cancer Cell Lines
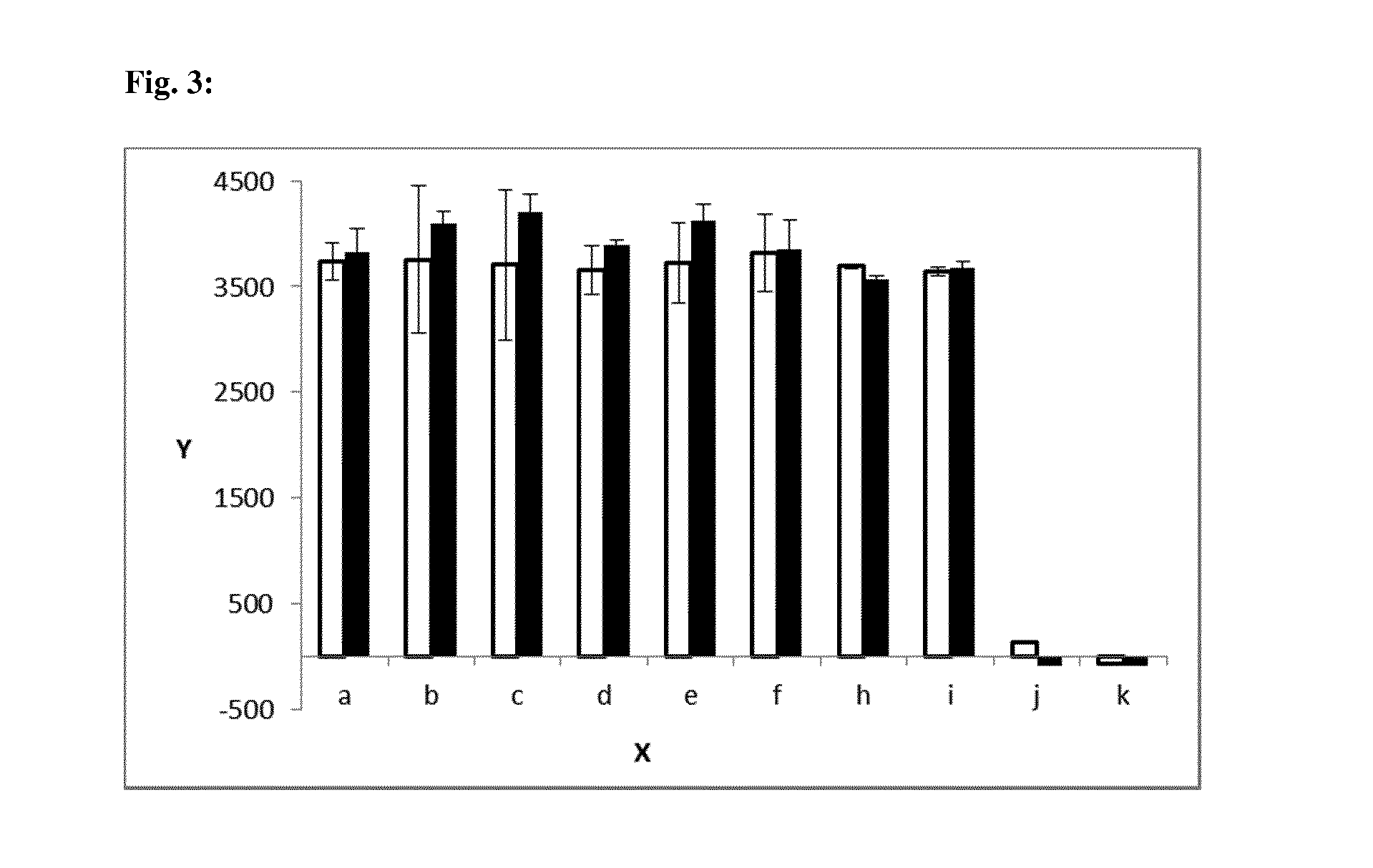
[0443]To determine the binding characteristics of the anti-TWEAKR antibodies on mouse and human cancer cell lines, binding was tested by flow cytometry to a panel of cell lines. Adherent cells were washed twice with PBS without Ca and Mg (Biochrom #L1825: aqueous solution containing 8000 mg / l NaCl, 200 mg / l KCl, 1150 mg / l Na2HPO4, and 200 mg / l KH2PO4) and detached by enzyme-free PBS based cell dissociation buffer (Invitrogen). Cells were suspended at approximately 105 cells / well in FACS buffer (PBS without Ca / Mg, containing 3% FCS, Biochrom). Cells were centrifuged (250 g, 5 min, 4° C.) and supernatant discarded. Cells were resuspended in dilutions of the antibodies of interest (10 μg / ml in 80 μl if not indicated otherwise) in FACS buffer, and incubated on ice for 1 h. In the following cells were washed once with 1000 cold FACS buffer and 80 μl secondary antibody diluted at 1:150 (PE goat anti-human IgG, Dianova #...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com