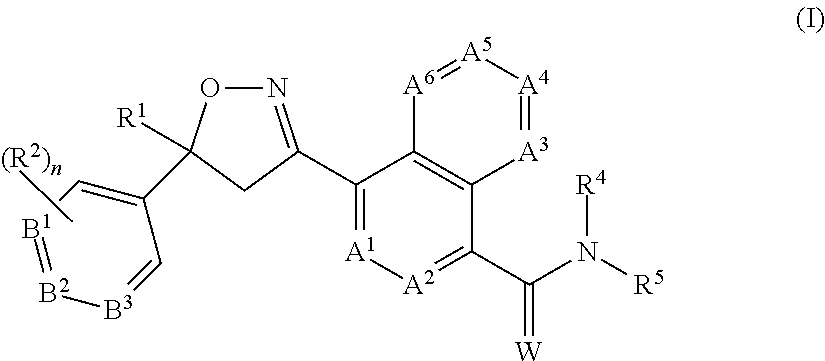
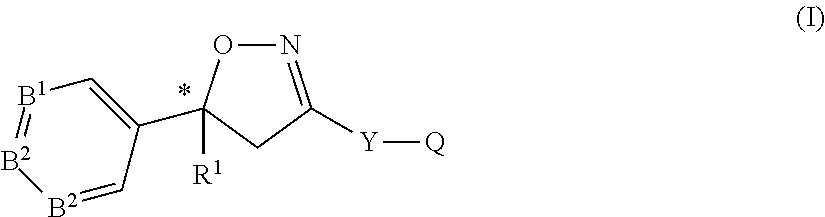
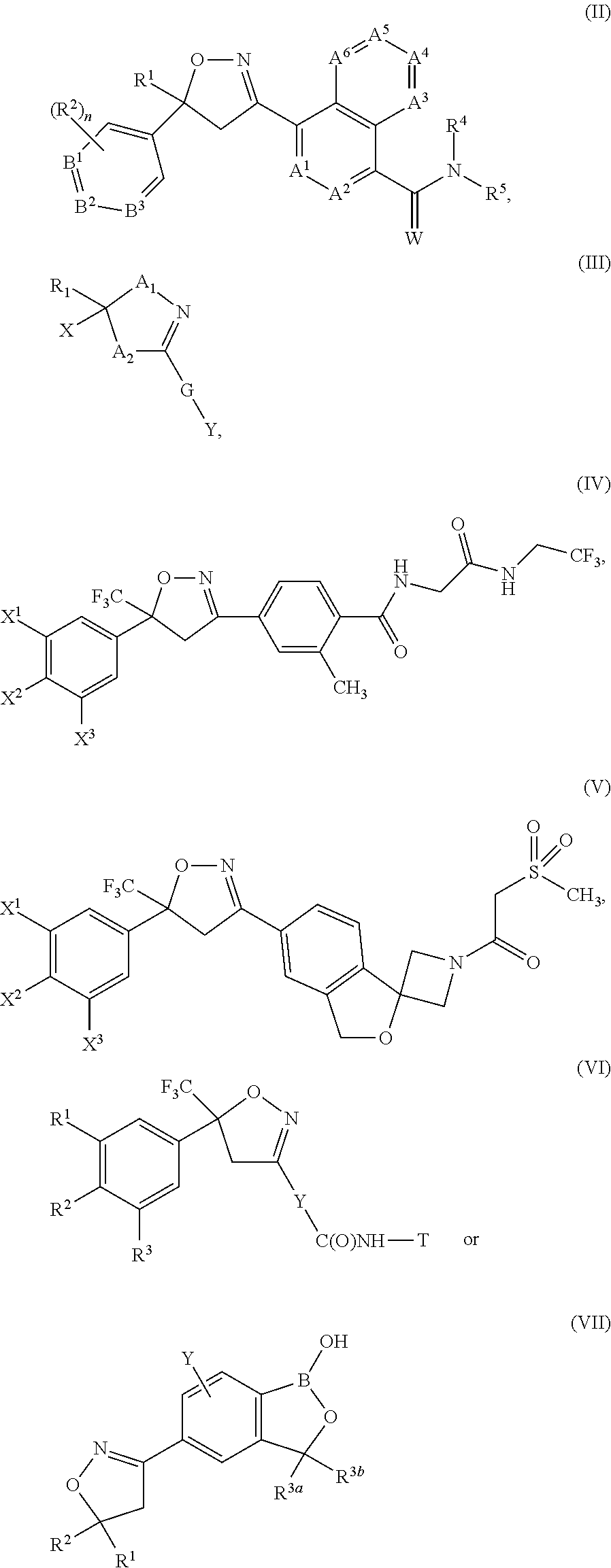
Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0853]The invention is further described by the following non-limiting examples which further illustrate the invention, and are not intended, nor should they be interpreted to, limit the scope of the invention.
formulation examples
[0854]The following extended release injectable formulations were prepared by mixing the following ingredients. Unless indicated otherwise, the concentrations of each component is percent (%) weight per weight (w / w) and MW refers to weight average molecular weight.
example 1
[0855]
Compound of formula (IIc) 26%Medium molecular weight (MMW) PLGA (50:50) 1%Propylene carbonate50.9%Triacetin22.2%BHT0.02%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com