Mapc treatment of brain injuries and diseases
a brain injury and disease technology, applied in the field of brain injury and disease treatment, can solve the problems of brain injury, including brain diseases, major health problems, focal hypoxia, cortical infarcts and strokes, etc., and achieve the effect of safe storag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hypoxic-Ischemic Injury with MAPCs in Rats and Treatment with MAPCs and Immunosuppression
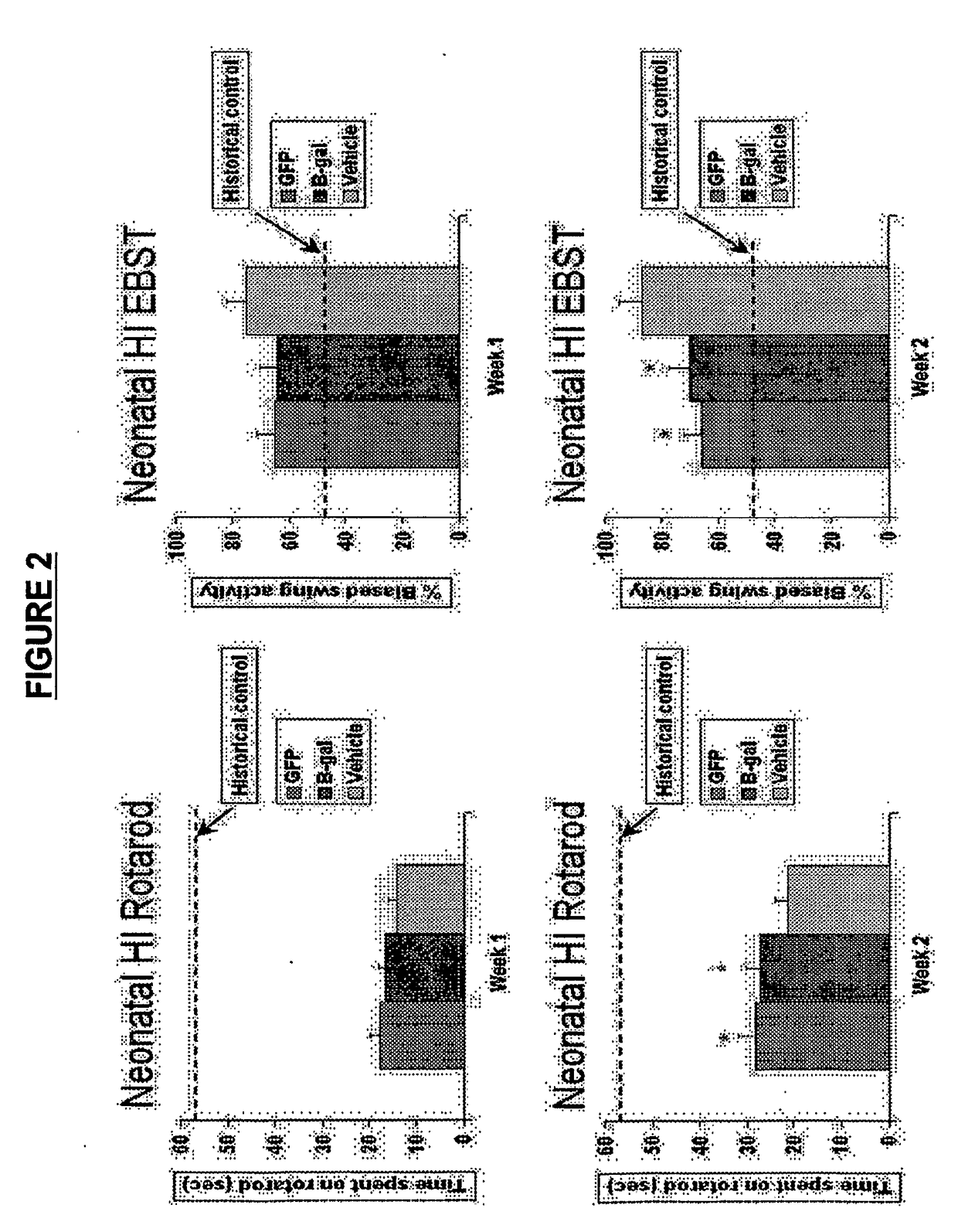
[0257]Seven day old Sprague Dawley (SD) rat pups (n=7 per test group) were subjected to HI injury by the method of unilateral carotid ligation followed by 8% hypoxia, as described in Rice et al., Ann Neural. 9: 131-141 (1981), which is herein incorporated by reference in its entirety particularly in regard to this method. Seven days after the injury, the animals underwent stereotaxic transplantation into the hippocampal region with cryopreserved MAPCs (thawed just prior to transplantation) derived from either SD rats (syngeneic, GFP-labeled, 200,000 cells per animal) or Fisher rats (allogeneic, β-gal-labeled, 200,000 cells per animal). All animals were treated with daily immunosuppression (CSA, 1 mg / kg, i.p.) throughout the survival period. On days 7 and 14 post-transplantation, the Elevated Body Swing Test (EBST) and Rotarod test were performed to reveal general and coordinated motor and neurol...
example 2a
Evaluation of Locomotor Skills at 7 and 14 Days After MAPC Injection in HI-Injury Rats
[0258]Animals were treated as described in Example 1. At day 7 post-transplantation, MAPC transplanted HI injured animals displayed a trend of less motor asymmetry as determined by the EBST (64%-65% versus 75%) and longer time spent on the rotarod (14.1-16.5 versus 18 seconds) compared to vehicle-infused injured animals. At day 14 post-transplantation, MAPC transplanted animals exhibited significantly reduced motor asymmetry (66%-70% versus 87%) and longer time spent on the rotarod (27.3-28.3 versus 21 seconds) than those control animals that received the vehicle infusion. Syngeneic and allogeneic MAPCs transplanted into injured animals did not differ statistically in their behavioral improvements at both test periods. Results are depicted graphically in FIG. 2. The results show the therapeutic effects of injected MAPCs in the rat HI injury model by both locomotor and neurological measures.
example 2b
Histological Analysis of MAPC Engraftment on Day 14 after MAPC Injection in Brains of HI Rats
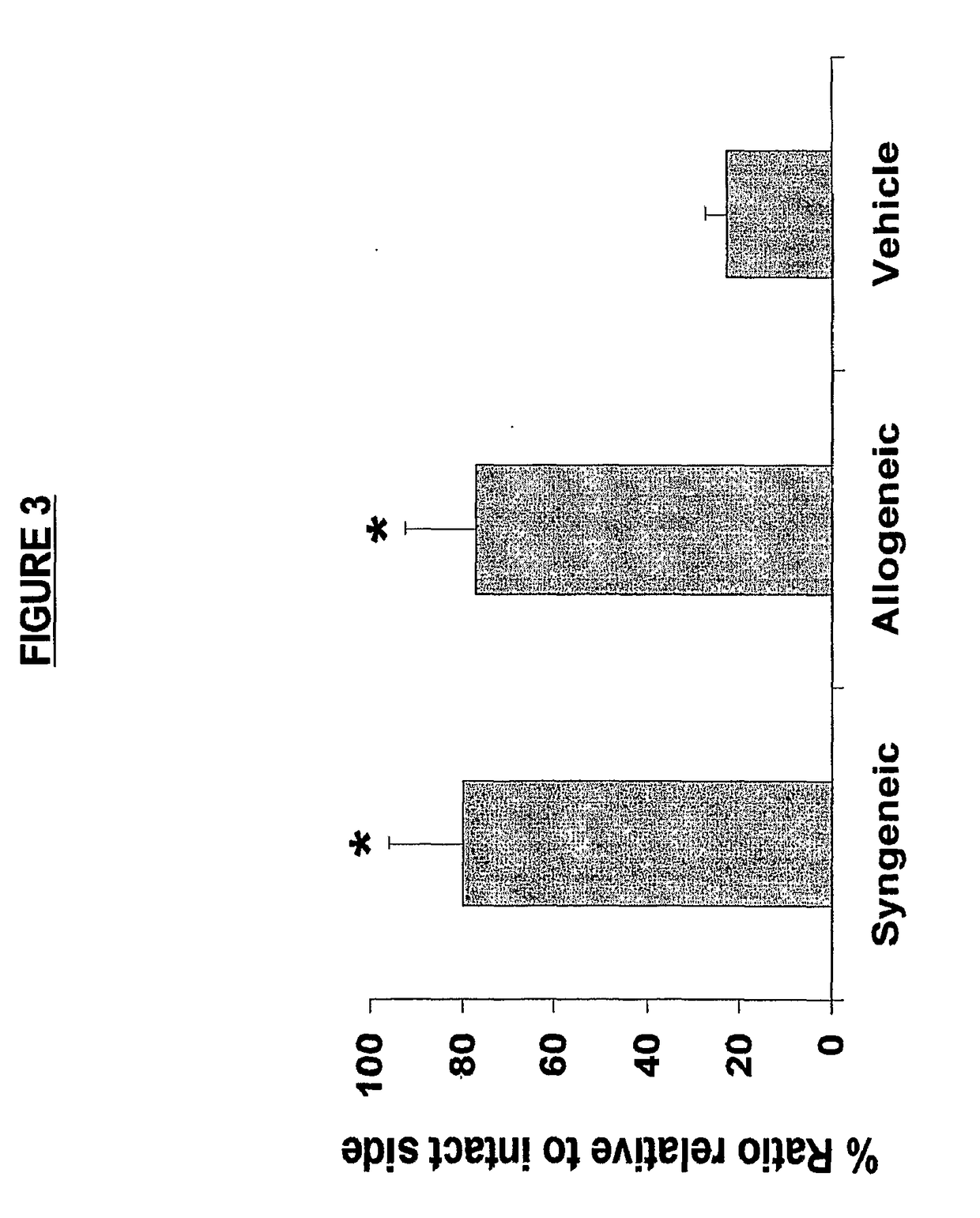
[0259]Animals were treated as described in Example 1. Grafted MAPCs were detected in the brains of the HI-injured animals after sacrifice on Day 14 post transplantation by histological examination. GFP-positive syngeneic grafts were detected mostly in the original hippocampal CA3 transplant site and adjacent CA2 region, which co-labeled with DAPI. Allogeneic grafts, detected by anti-β-gal staining and co-labeling with DAPI, displayed a similar pattern of graft survival in HI injured brains. Graft survival was 0.96% at 14 days (ANOVA F value is 24.27, df=2, 19 and p<0.0001; Fisher posthoc is p<0.0001). The results show that both allogeneic and syngeneic MAPCs engraft at the injection site and persist to at least two weeks after direct intracerebral injection in animals in the rat HI injury model.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| cell mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com