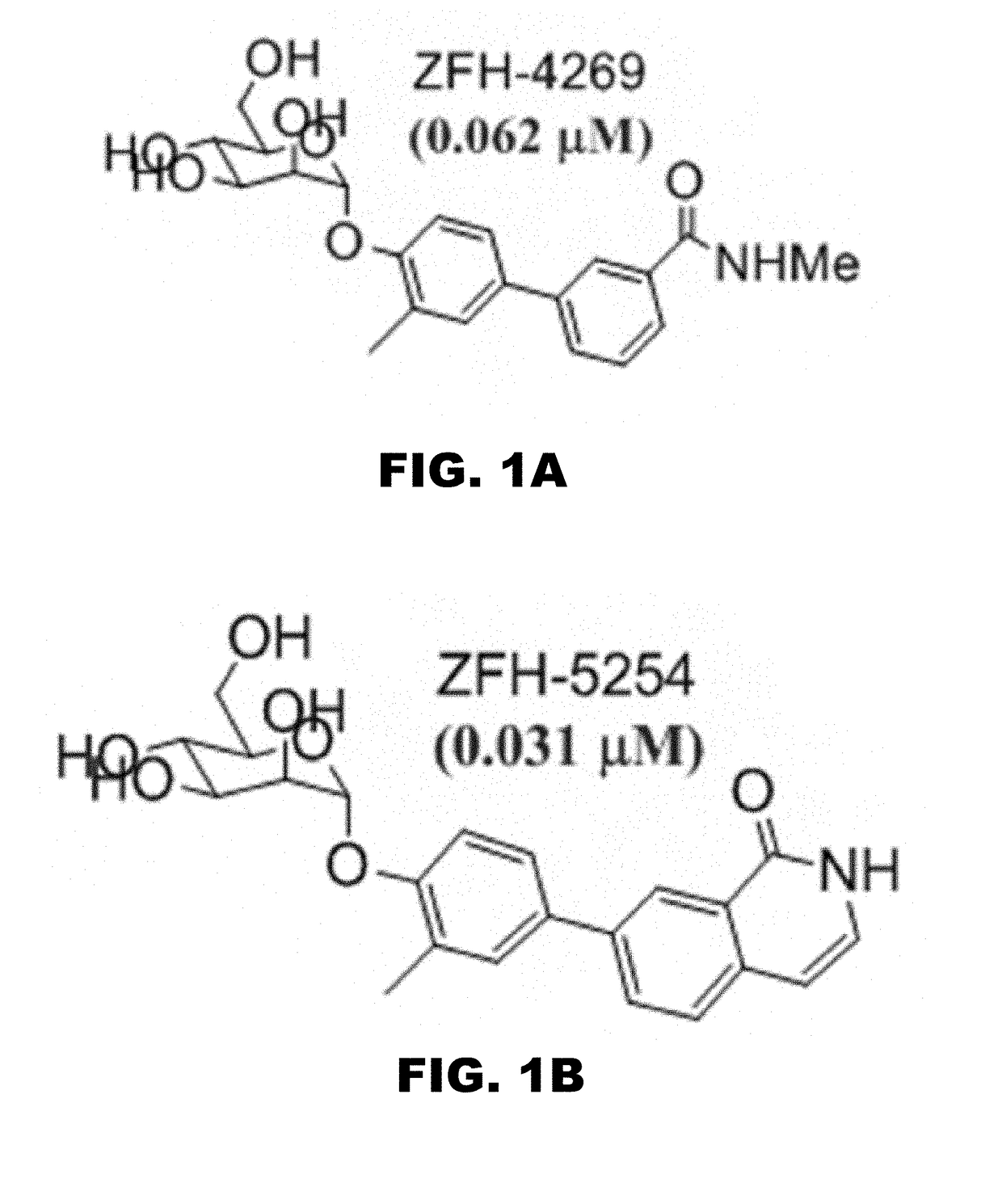
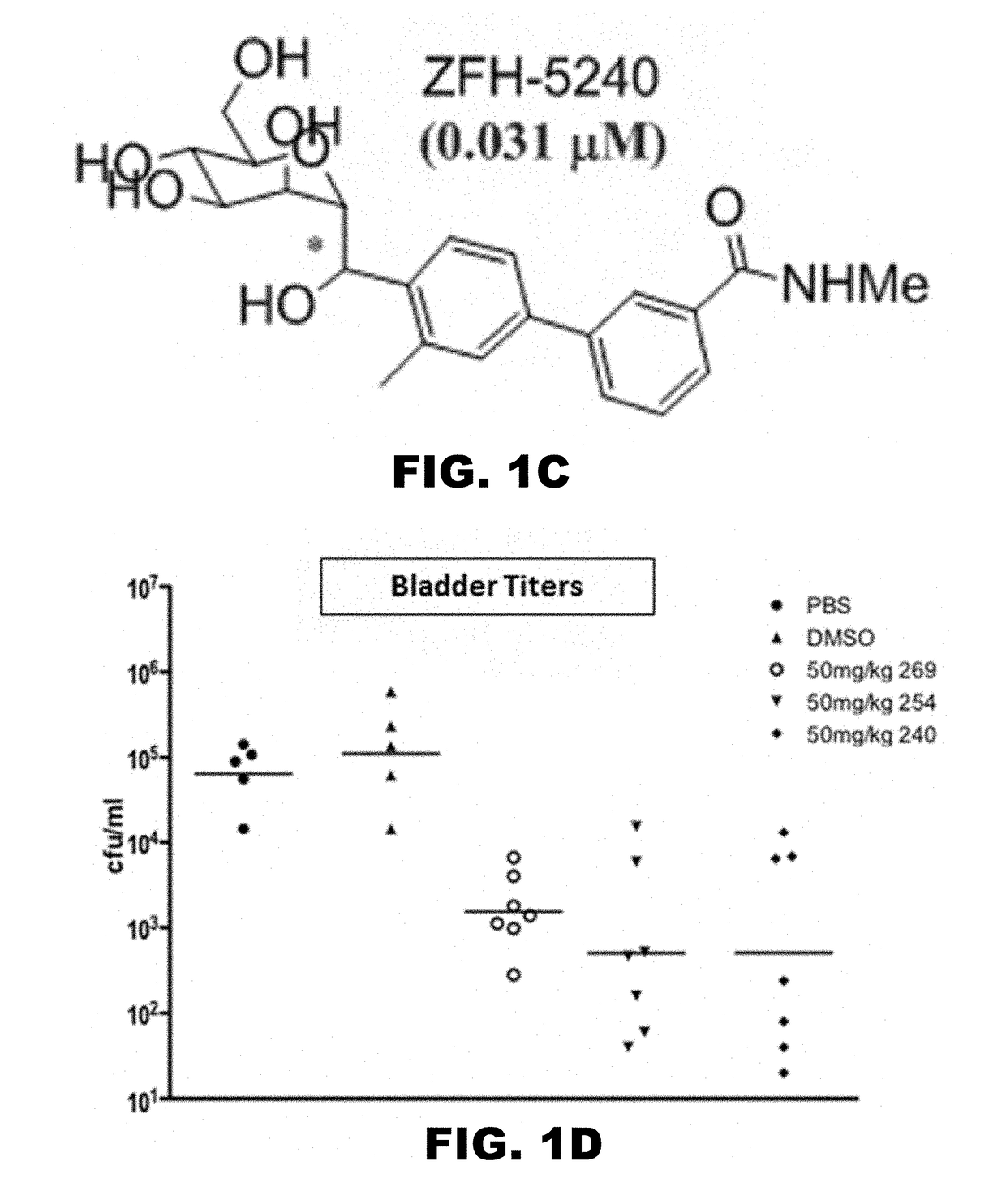
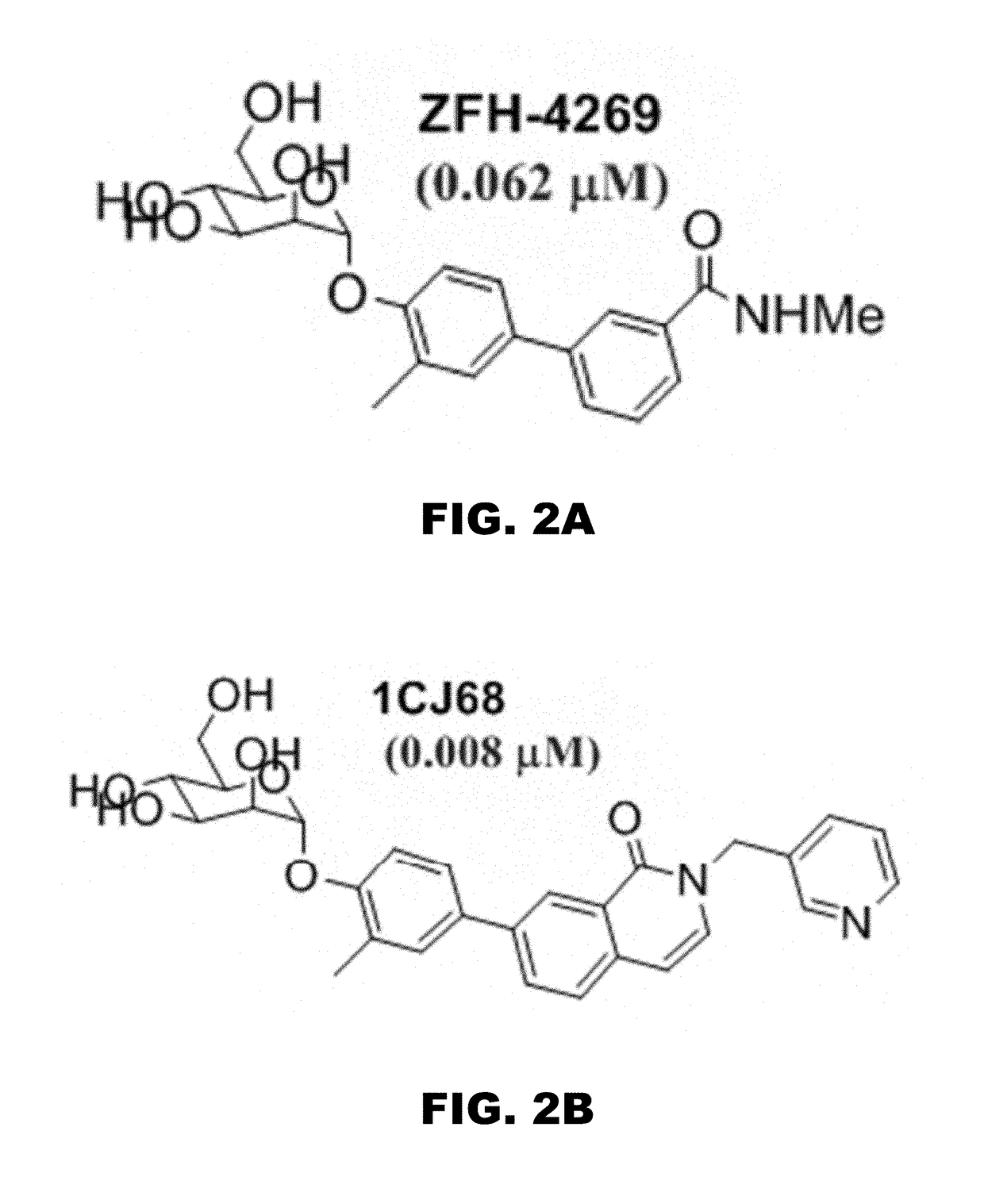
Compounds and methods for treating bacterial infections
a technology for bacterial infections and compounds, applied in the field of compounds and methods for treating bacterial infections, can solve the problems of serious recurrence and significant problems in recurrent infections, and achieve the effect of reducing the resistance of a bacterium and preventing a urinary tract infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[3-methyl-4-[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzoate
[0684](Han et. al., J. Med. Chem. 2012, 55, 3945-3959).
[0685]To a round-bottomed flask equipped with a reflux condenser and N2 line was added [(2R,3R,4S,5R,6S)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-methyl-phenoxy)tetrahydropyran-3-yl] acetate (0.52 g, 1.0 mmol), (3-methoxycarbonylphenyl)boronic acid (0.22 g, 1.2 mmol), Cs2CO3 (0.98 g, 3 mmol) and Pd(Ph3)4 (0.12 g, 0.1 mmol) followed by 5:1 mixture of 1,4-dioxane / water (30 mL). The reaction flask was placed under high vacuum and then repressurized with N2 repeated 3 times. The reaction was heated to 80° C. under a N2 atmosphere for 1 h. The solvent was removed in vacuo and the residue was dissolved in CHCl3 and filtered. The filtrate was purified by silica gel chromatography (ISCO MPLC, MeOH / CH2Cl2, 0-10% gradient). Pure fractions as determined by TLC and LCMS were combined and then concentrated in vacuo. The residue was dis...
example 2.3
Example 2. 3-[3-methyl-4-[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzoic acid
[0686]To a solution of methyl 3-[3-methyl-4-[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzoate (0.222 g, 0.55 mmol) in MeOH (70 mL) was added 0.2 M NaOH (30 mL). The reaction was stirred overnight at RT. DOWEX 50WX4-100 ion exchange resin was added. After 15 minutes, the resin was filtered, washed with MeOH and then the filtrate was concentrated in vacuo to yield the title compound (0.2025 g, 94%) as a white solid. LCMS (ESI, M+Na+=413.3); 1H NMR δ ppm (d3-MeOD; 2.31 (s, 3H) 3.61 (ddd, J=9.78, 5.09, 2.74 Hz, 1H) 3.69-3.84 (m, 3H) 3.97 (dd, J=9.39, 3.52 Hz, 1H) 4.08 (dd, J=3.33, 1.76 Hz, 1H) 5.56 (d, J=1.96 Hz, 1H) 7.31 (d, J=8.22 Hz, 1H) 7.39-7.48 (m, 2H) 7.51 (t, J=7.83 Hz, 1H) 7.76-7.84 (m, 1H) 7.95 (dt, J=7.83, 1.37 Hz, 1H) 8.21 (t, J=1.76 Hz, 1H)).
example 3.3
Example 3. 3-[3-methyl-4-[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]-N-(4-pyridyl)benzamide
[0687]To a stirred solution of 3-[3-methyl-4-[(2R,3R,4S,5S,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzoic acid (0.039 g, 0.1 mmol) and HATU (0.046 g, 0.12 mmol) in DMF (5 mL) under a N2 atmosphere and cooled to 0° C. was added 4-aminopyridine (0.011 g, 0.12 mmol), and DIPEA (0.054 mL, 0.3 mmol). The reaction was allowed to warm to RT and then stirred overnight. The solvent was removed in vacuo and the residue purified by reversed phase HPLC (5-85% acetonitrile / water / 0.05% TFA). Pure fractions were combined and lyophilized to give the title compound as a white powder (0.047 g, 100%). LCMS (ESI, M+H+=467.3); 1H NMR δ ppm (d3-MeOD; 2.34 (s, 3H) 3.60 (ddd, J=9.78, 5.28, 2.54 Hz, 1H) 3.68-3.85 (m, 3H) 3.98 (dd, J=9.59, 3.33 Hz, 1H) 4.09 (dd, J=3.33, 1.76 Hz, 1H) 5.58 (d, J=1.57 Hz, 1H) 7.34 (d, J=8.61 Hz, 1H) 7.44-7.58 (m, 2H) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com