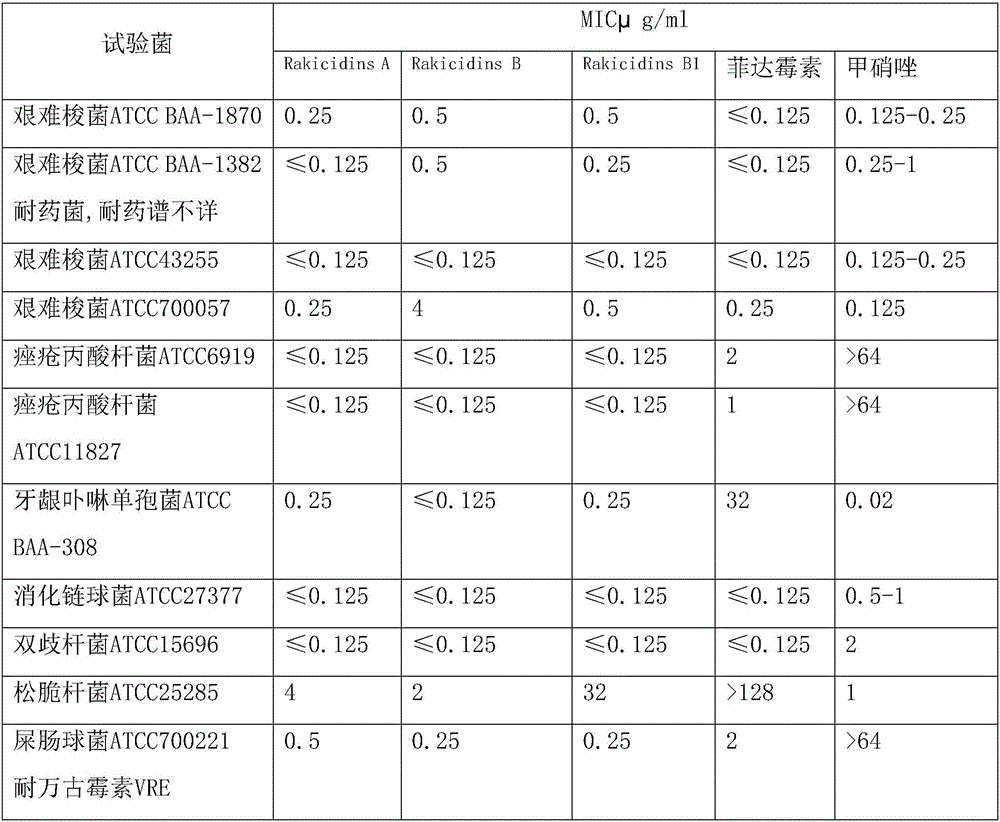
Application of Rakicidins compounds in resistance to clinical pathogenic anaerobic bacteria
A technology of compound and anaerobic bacteria, applied in the field of Rakicidins compound, anti-clinical pathogenic anaerobic bacteria, can solve the problem of high recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
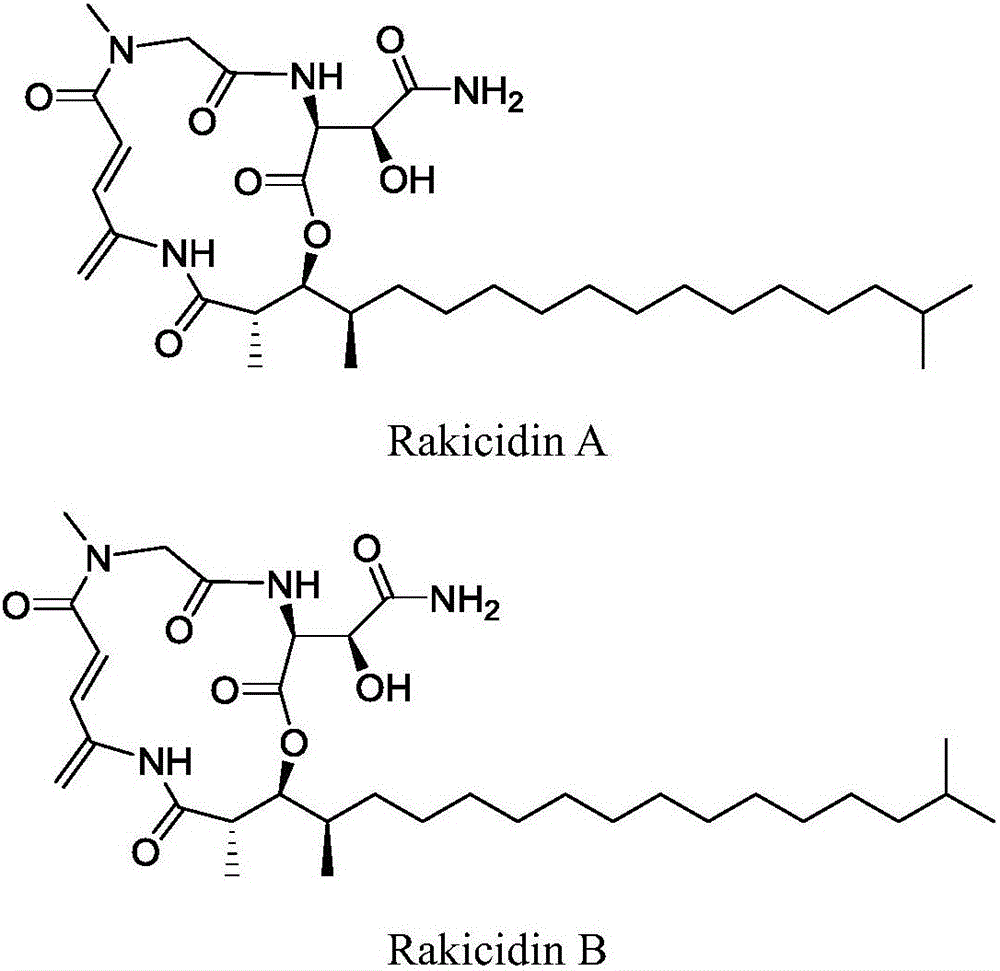
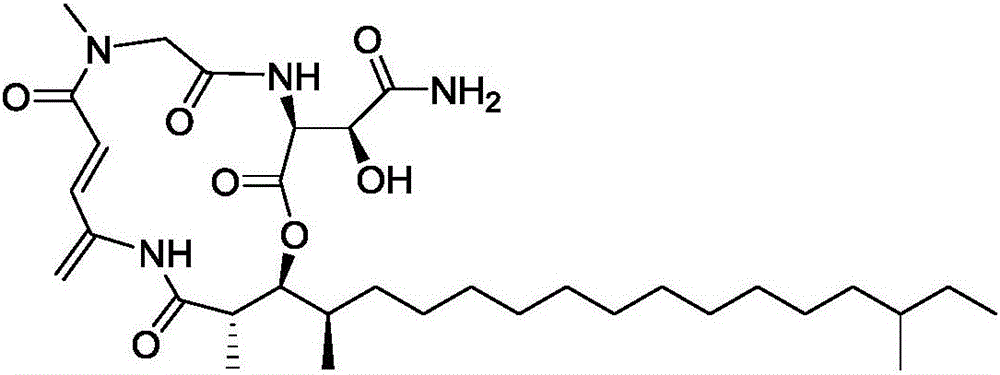
[0022] Preparation of RakicidinB1
[0023] Step 1: The fermentation and culture conditions of Micromonospora strain FIM02-523 refer to the literature (Jiang Hong, Lin Ru, Zheng Wei, etc. The separation, identification and biological biological activity [J]. Chinese Journal of Antibiotics, 2006, 31(5): 267-270).
[0024] Step 2: Centrifuge the FIM02-523 fermentation broth obtained in Step 1 at 4500rpm for 15 minutes to obtain the mycelium residue, soak the obtained mycelium residue with 2 times the volume of anhydrous methanol or ethanol overnight, and sterilize the bacteria containing alcohol The silk residue was centrifuged again at 4500 rpm for 15 minutes, and the supernatants were combined to obtain a fermentation extract.
[0025] Step 3: HP20 macroporous resin adsorption column chromatography (diameter-to-height ratio 1:5-1:10, column volume 1.5-2.5L): use fermentation extract (40-60L) with 50%-55% Alcohol concentration, the flow rate is 40ml / min for upper column adsorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com