Motile Sperm Domain Containing Protein 2 and Inflammation
a technology of sperm domain and protein, applied in the field of antiinflammatory processes, can solve the problems of phase ii clinical trial failure of chemokine and chemokine receptor antagonists, and achieve the effects of treating, preventing, inhibiting or preventing one or more activities, and preventing or reducing the incidence of an inflammatory disease or disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MOSPD2 and Chemokine-Induced Monocyte Migration
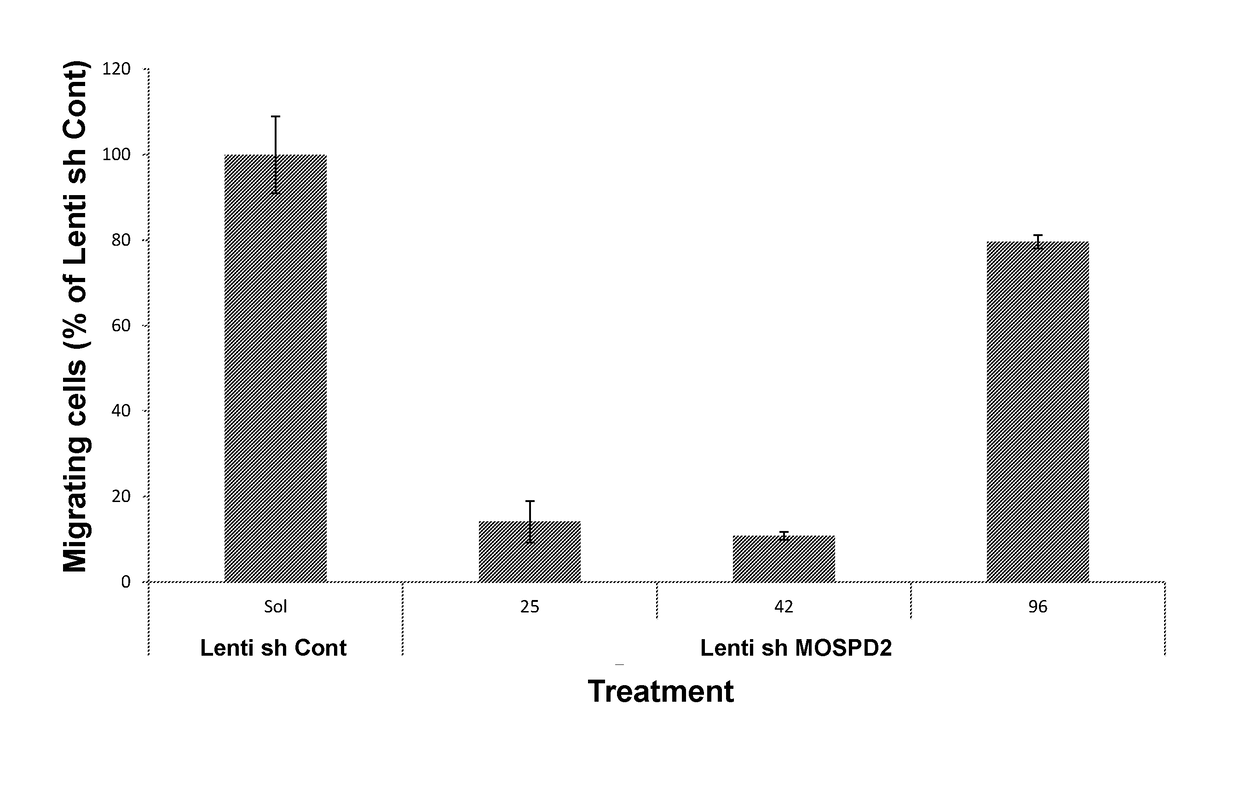
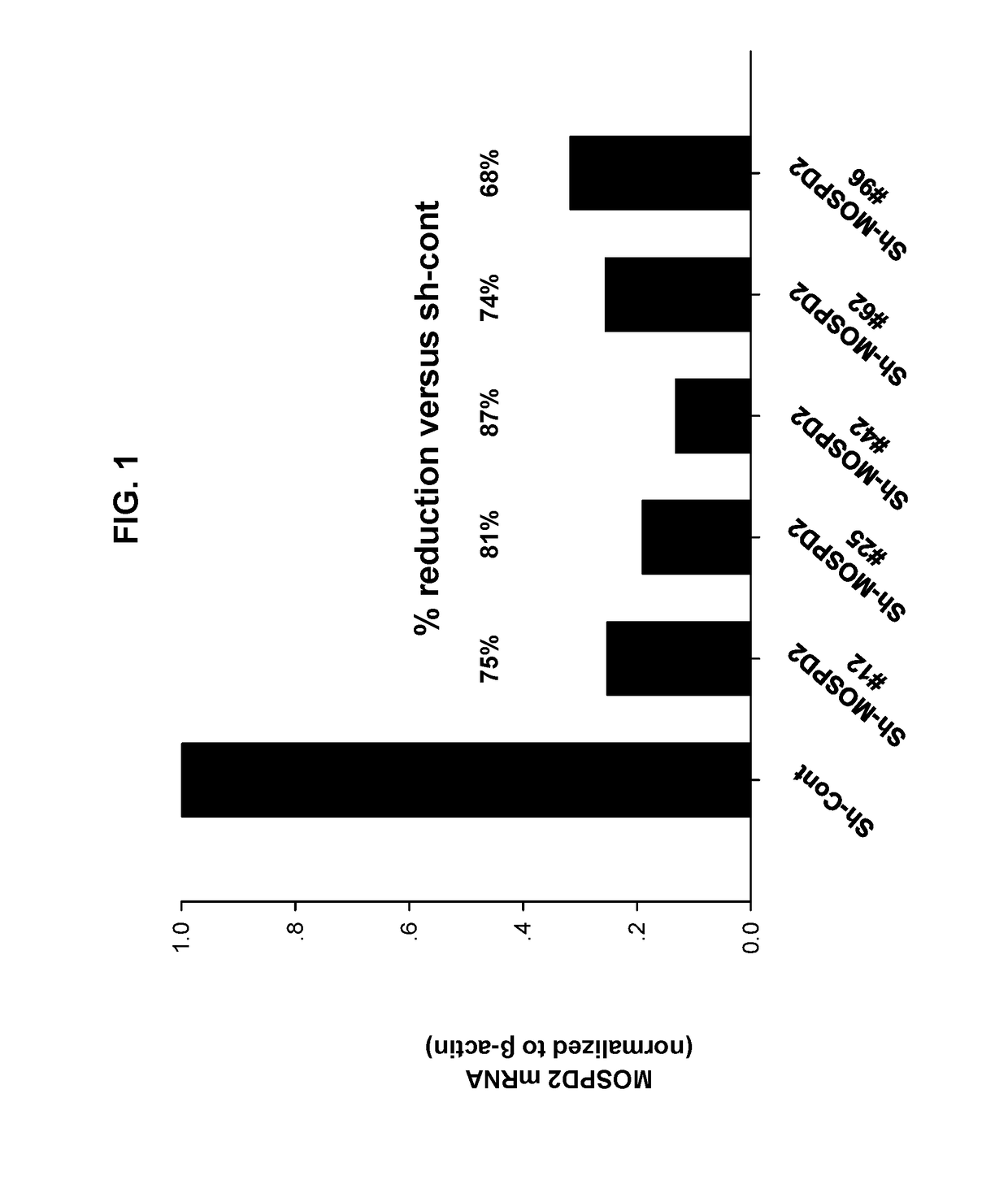
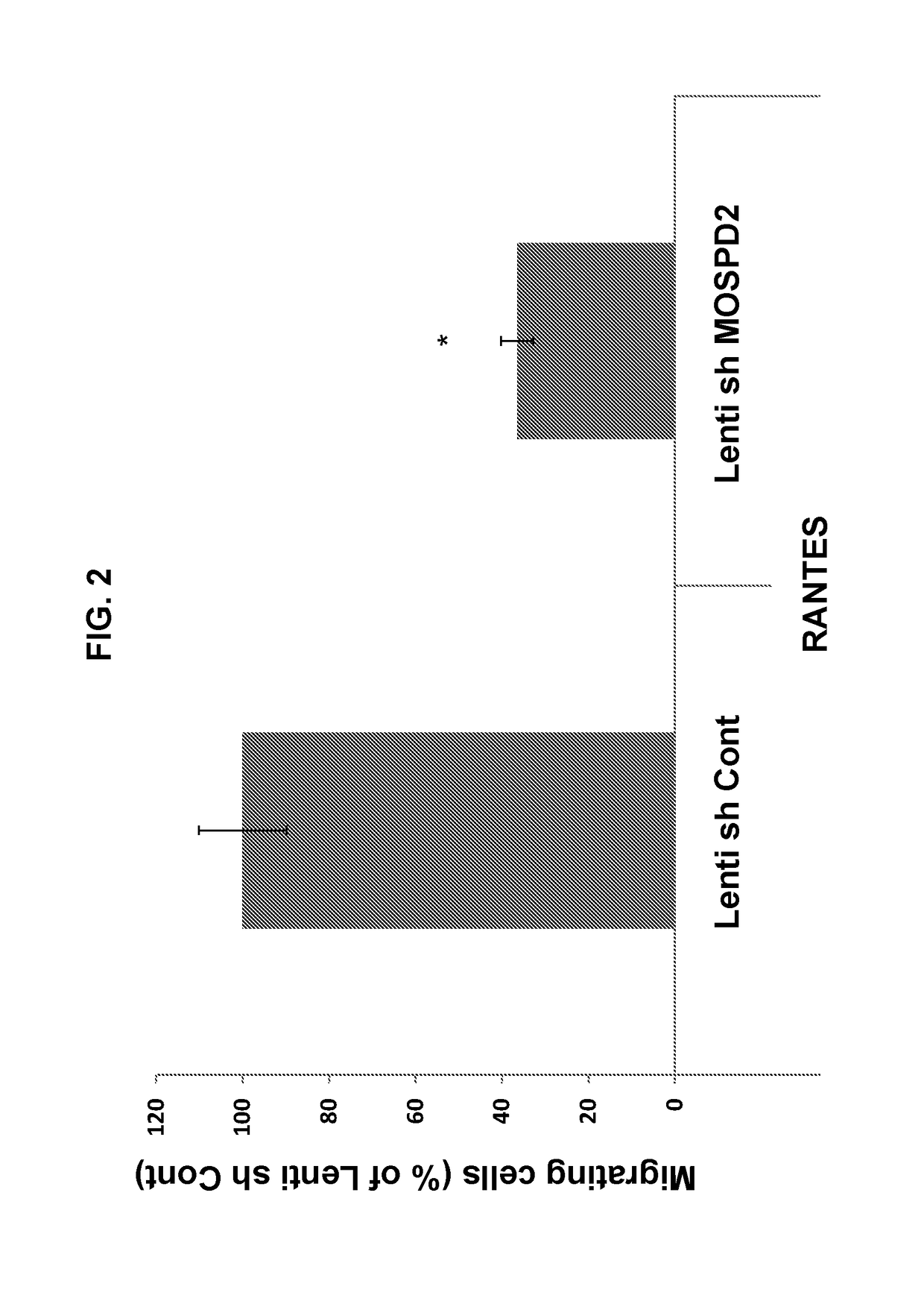
[0322]To assess the role of MOSPD2 in monocyte migration, MOSPD2 expression was silenced in U937 cells as described in the Materials and Methods section using Lenti-virus particles containing sh-RNA directed against three different regions of MOSPD2 mRNA (sh-MOSPD2). MOSPD2 mRNA expression in the cells was assessed using quantitative PCR (Q-PCR) and normalized to β-actin expression as control. FIG. 1 shows that all tested sh-MOSPD2 profoundly reduced mRNA expression levels of human MOSPD2. U937 cells transduced with control Lenti-virus particles or with sh-MOSPD2 Lenti-virus particles were then tested for migration towards the chemokine, RANTES, using a trans-well migration assay. FIGS. 2 and 3 show that cell migration induced by RANTES was significantly inhibited in sh-MOSPD2 transduced cells compared to cells in which MOSPD2 was not silenced.
example 2
MOSPD2,ERK Phosphorylation and AKT Phosphorylation
[0323]The effect of MOSPD2 inhibition on the activation of chemokine-induced signaling pathways was determined by testing the effects of MOSPD2 inhibition on phosphorylation of ERK and AKT. U937 cells transduced with control Lenti-virus particles or sh-MOSPD2 Lenti-virus particles were treated with RANTES for 2 or 5 minutes and then analyzed by western blot for phosphorylated ERK and AKT. Heat shock protein 90 (HSP90) was used as a loading control. FIG. 4 shows that inhibition of MOSPD2 almost completely abolished RANTES-induced phosphorylation of ERK and AKT.
example 3
MOSPD2 and Chemokine Receptor-Driven Signaling
[0324]The effect of MOSPD2 inhibition on other chemokines was also tested. U937 cells transduced with control Lenti-virus particles or sh-MOSPD2 Lenti-virus particles were tested for migration towards MCP-3, MCP-1, RANTES and SDF-1 in a trans-well assay and for levels of phosphorylated ERK and AKT by western blot. FIG. 5 shows that MOSPD2 inhibition significantly inhibited cell migration induced by all the tested chemokines. Furthermore, FIG. 6 shows that inhibition of MOSPD2 almost completely abolished phosphorylation of ERK and AKT induced by all the tested chemokines. As such, the effects of MOSPD2 inhibition on migration and signaling are not limited to a single chemokine or chemokine receptor pathway.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com