Tubulin-binding compounds, compositions and uses related thereto
a technology of tubulin and compound, applied in the field of compound, can solve problems such as important dose-limiting toxicities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Protocols
[0186]Chemistry: General Experimental Procedures.
[0187]All reagents and solvents were purchased from commercial suppliers and used without further purification unless otherwise stated. The reactions were monitored by thin layer chromatography (TLC) on precoated silica gel F254 plates (Sigma-Aldrich) with a UV indicator using chloroform:methanol (9.5:0.5 v / v). Yields were of purified product and were not optimized. The purity of the newly synthesized compounds was determined by LCMS analysis. The proton nuclear resonance (1H NMR) spectra were performed on a Varian GEMINI 2000 NMR spectrometer system with working frequency 400 MHz. Chemical shifts (δ) are given in ppm, and the following abbreviations are used: singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad singlet (br s). All LCMS data were gathered on an Agilent 1100 LC system. The compound solution was injected into the ionization source (APCI) operating positive and negative modes wi...
example 2
-Activity Relationship Studies for Certain Compounds of the Invention
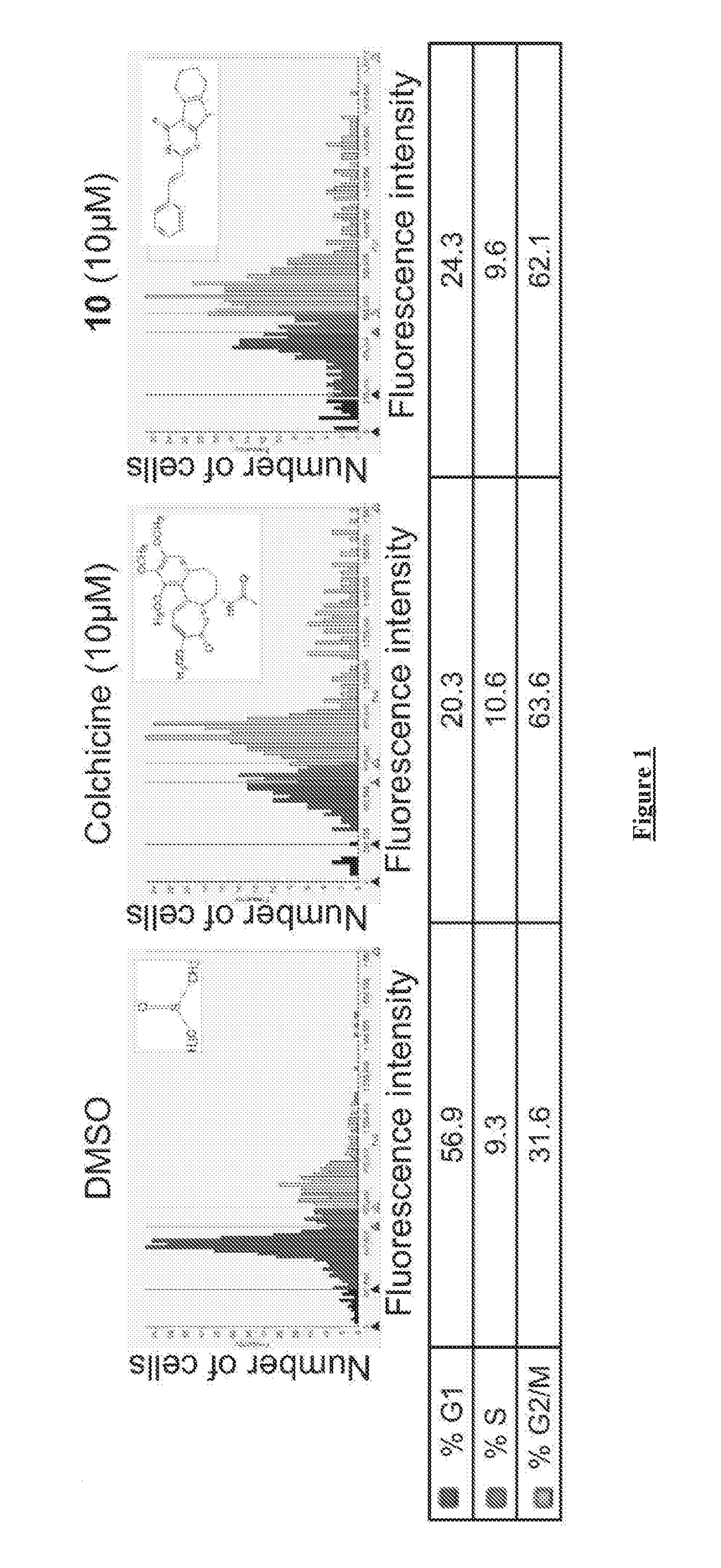
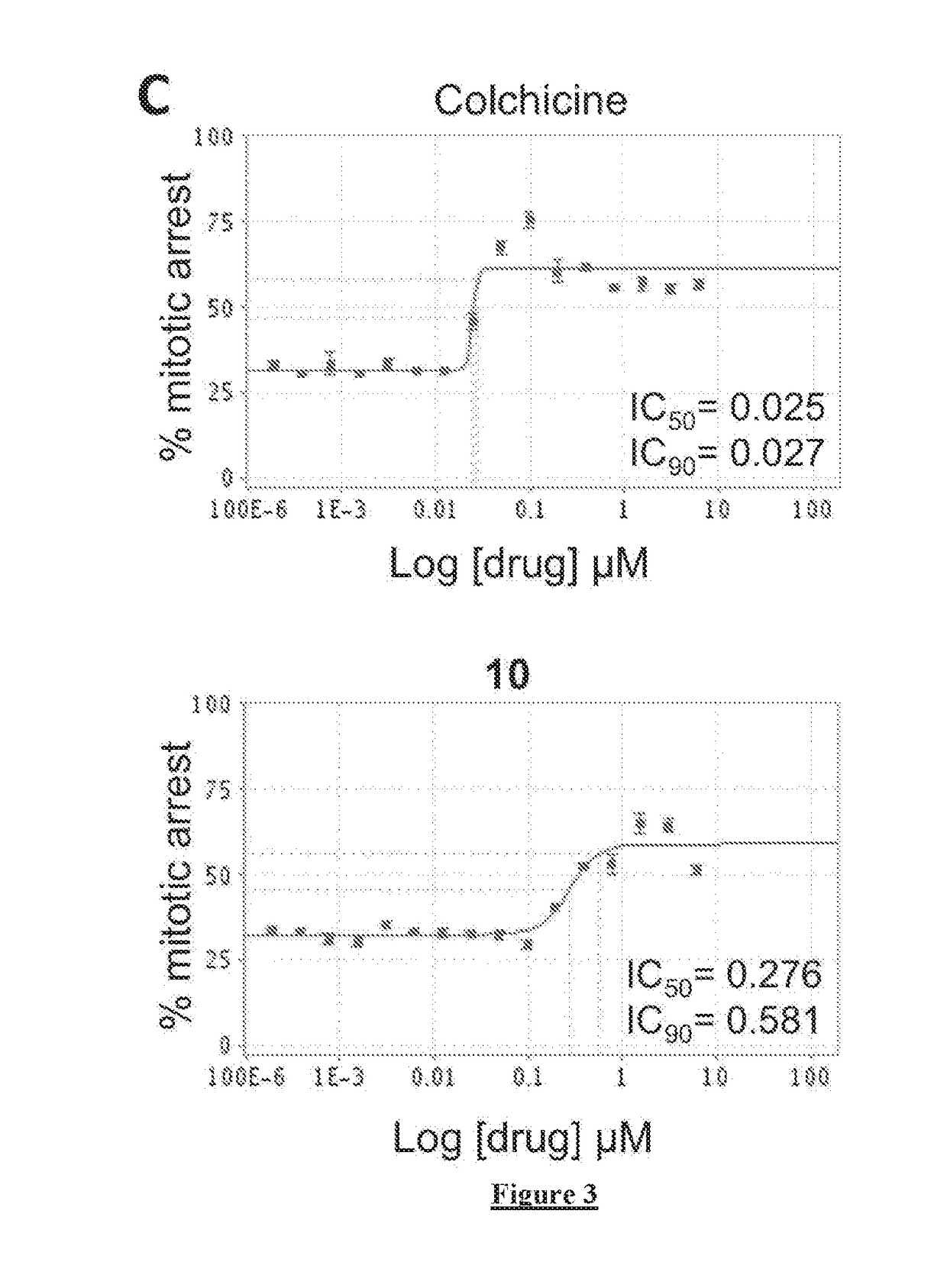
[0208]The cell viability IC50 of each compound was determined using Promega CellTiter-Glo Luminescent Cell Viability Assay kit by measuring the total ATP levels to quantify the number of metabolically active cells upon drug treatment as described in Crouch et al., J Immunol Methods 1993, 160, (1), 81-8. Briefly, the compounds were suspended in DMSO at 10 mM and diluted in 384 plates (20 μl / well in DMSO) in triplicate by a 14-point titration (12 nM to 100 μM). 50 μl of HeLa cells or patient derived glioblastoma cells (HK-309) (2000 cells / well) were then treated with the prepared dilutions of the drugs (0.5 μl) and incubated at 37° C. and 5% CO2. 72 hours later 50 μl of CellTiter-Glo reagent was added to each well followed by a 2 minutes shaking and a 10-minute incubation to lyse the cells. The relative luminescent intensity units (RLU) of each well was measured using a Tecan M1000 microplate reader (Tecan Group Ltd....
example 3
le Polymerization Assays
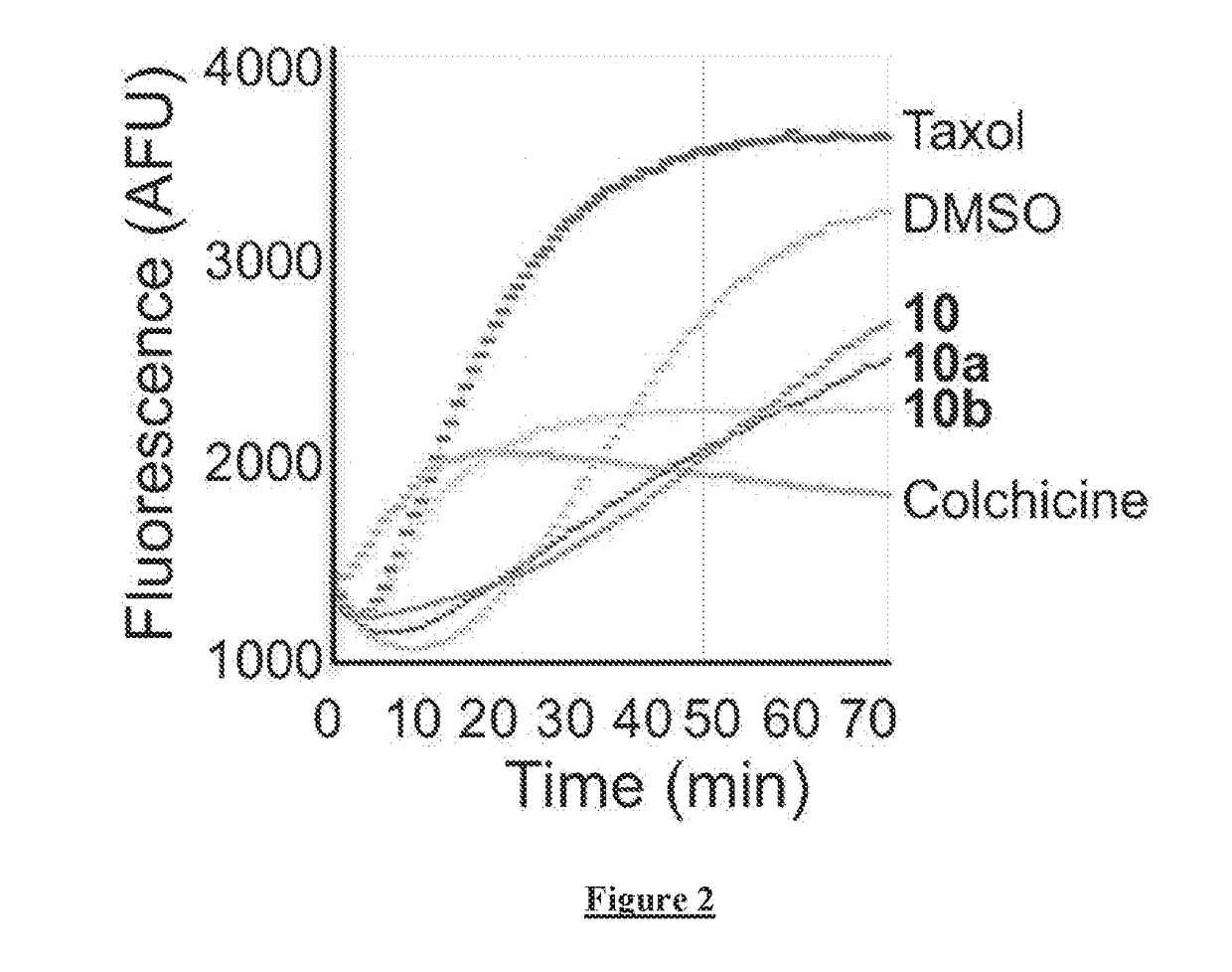
[0211]To further validate that compound 10 and its analogues were targeting microtubules, in vitro microtubule polymerization reactions were performed with the most potent compounds (10-10b) using the HTS-tubulin polymerization assay kit from Cytoskeleton Inc. Briefly, tubulin polymerization assays were conducted using the HTS-Tubulin polymerization assay kit from Cytoskeleton Inc. The reactions were carried out according to the manufacturer instructions (Cytoskeleton, BK011P) in the presence of 3 μM of test compounds (10, 10a, and 10b) and controls (DMSO, colchicine and taxol). 1.5 ul of 10X strength compound, 20 μl of tubulin solution and Triton X-100 at a final concentration of 0.01% were added to each well in a 384 well plate. The reactions were assembled on ice to prevent tubulin pre-polymerization. For kinetic measurements, microtubule polymerization was monitored by reading the fluorescence at 420 nm (due to the incorporation of a fluorescent reporter ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com