Formulations and methods for the treatment of cancers
a cancer and cancer technology, applied in the field of cancer treatment methods, can solve the problems of not leading to clinically acceptable remission, the origin of castration resistant prostate cancer cells or the interaction between heterogeneous subpopulations of prostate cancer cells contributing to castration resistant prostate cancer remains poorly understood, and the treatment of adt has not been successful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Patterns of AR Expression in Clinical Castration-Resistant Prostate Cancer and Castration-Resistant (CR) Xenografts
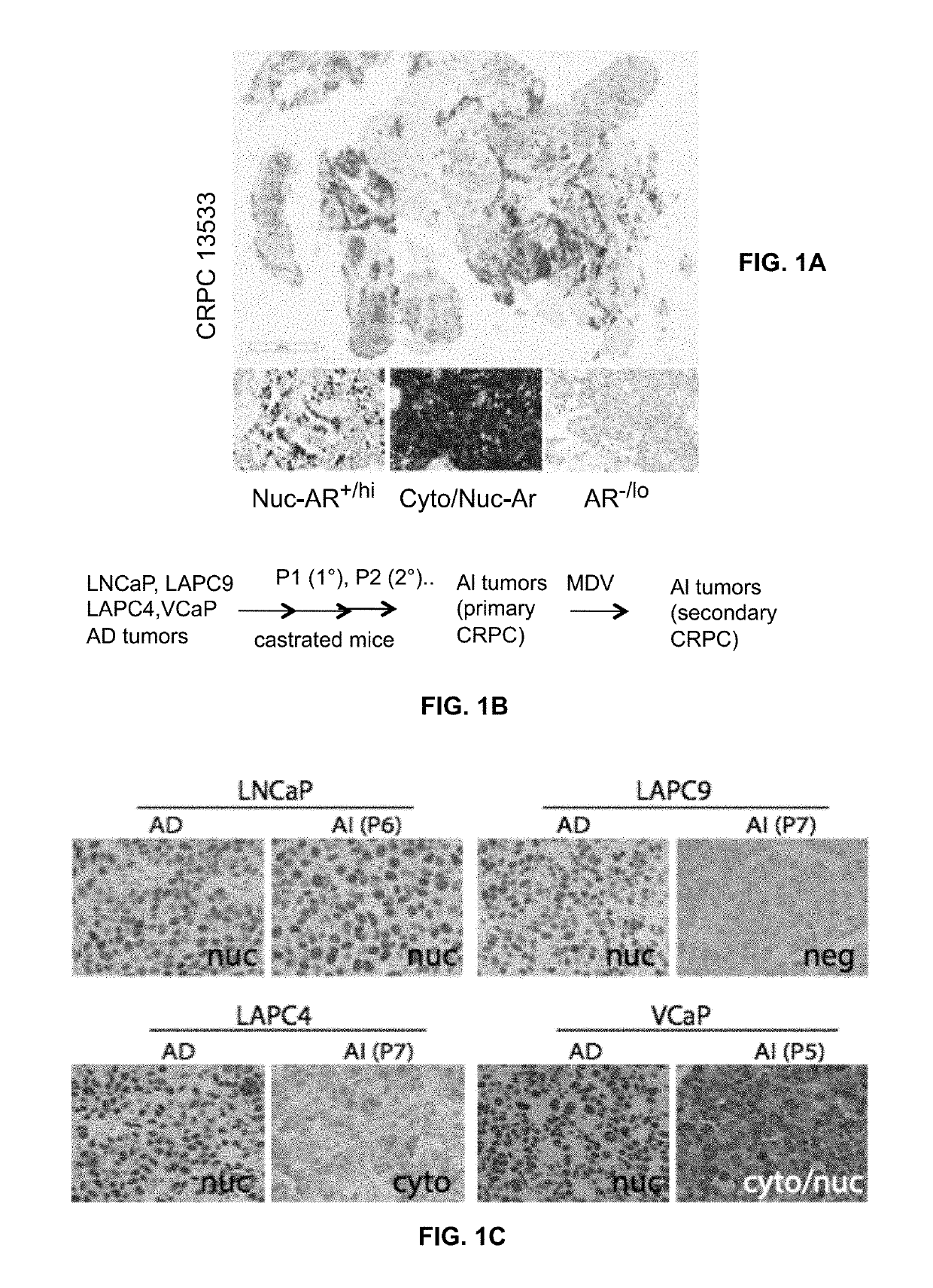
[0044]About 100 castration resistant prostate cancer whole-mount (WM) or tissue microarray (TMA) slides were assayed for androgen receptor expression using two antibodies that recognize the N-terminal epitopes of the androgen receptor, which could detect both full-length androgen receptor and all c-terminal truncated androgen receptor variants. The results revealed interesting and striking heterogeneous patterns of androgen receptor expression. Of the tested clinical samples, 26 cases (25%) completely lacked androgen receptor expression. The remaining AR+ tumors showed highly heterogeneous androgen receptor expression with both AR+ and AR− / lo areas either inter-mingled with or frequently separated from each other (FIG. 1A). When ‘zoomed’ in, the androgen receptor-positive PCa cells showed 3 expression patterns: substantially nuclear (nuc-AR), substantially cytoplasmic (...
example 2
Molecular Changes in Secondary LNCaP Castration Resistant Prostate Cancer in Response to Enzalutamide Therapy
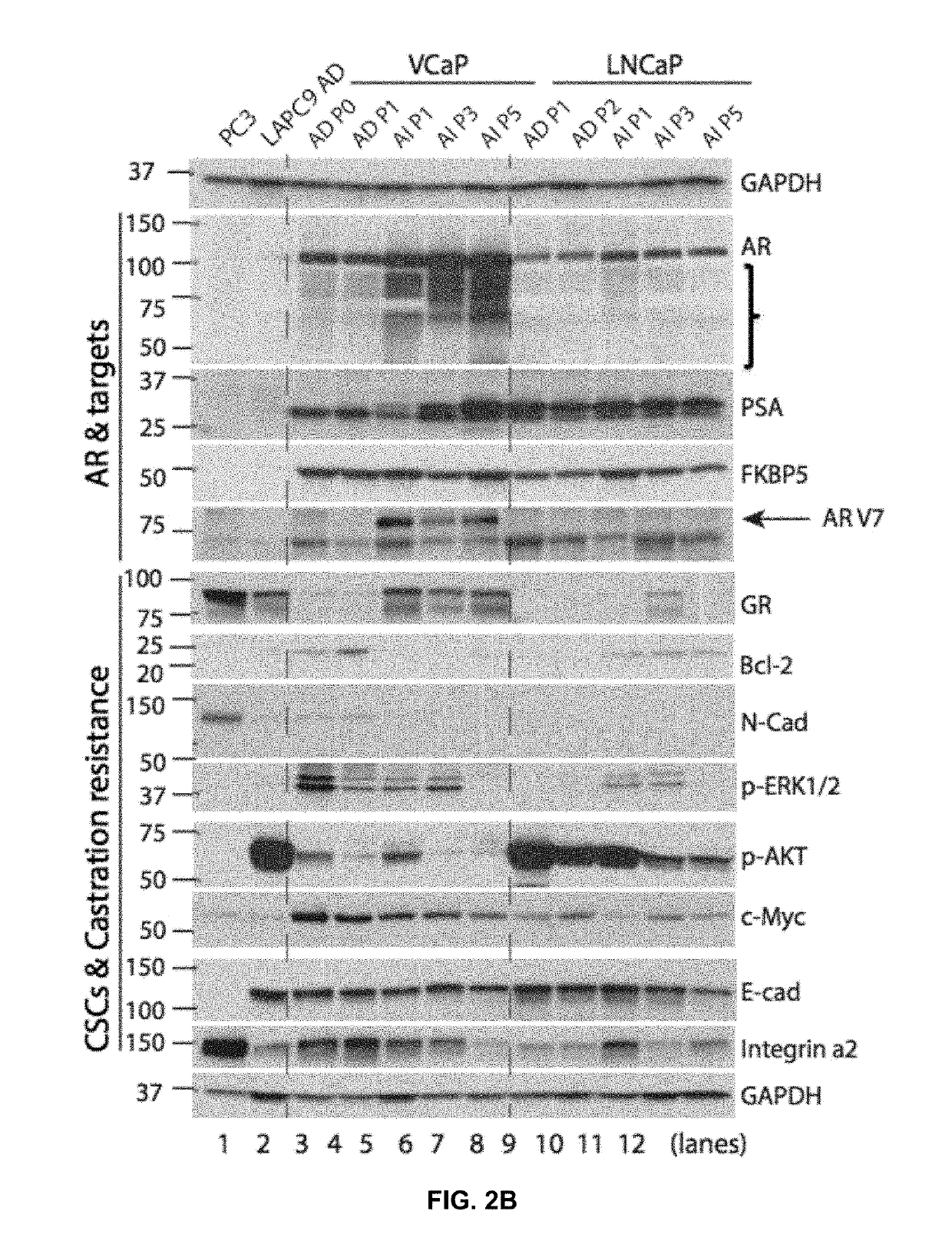
[0047]To test the efficacy of enzalutamide in reducing androgen-independent PCa tumor burden, therapy experiments were performed by treating mice bearing LNCaP AI-tumors with enzalutamide, administered by i.p. route. FIG. 4B revealed that enzalutamide suppressed growth of androgen-independent LNCaP for the first 6.5 weeks, suggesting that the upregulated nuc-AR (see FIG. 1C) is causally mediating the primary castration resistant prostate cancer in the LNCaP androgen-independent-tumor model. However, at about 7 weeks no further response to enzalutamide was observed (arrow, FIG. 4B) suggesting the emergence of enzalutamide-resistant tumors. These secondary castration resistant prostate cancer tumors were resistant to both surgical castration and enzalutamide as evidenced by western blotting analysis (FIG. 4C) and immune-histochemical analysis (FIG. 4D) for markers, androgen rec...
example 3
Castration of LAPC9 AD Tumors Leads to Decreased AR and Upregulation of Many CSC and Castration-Associated Molecules and Pathways
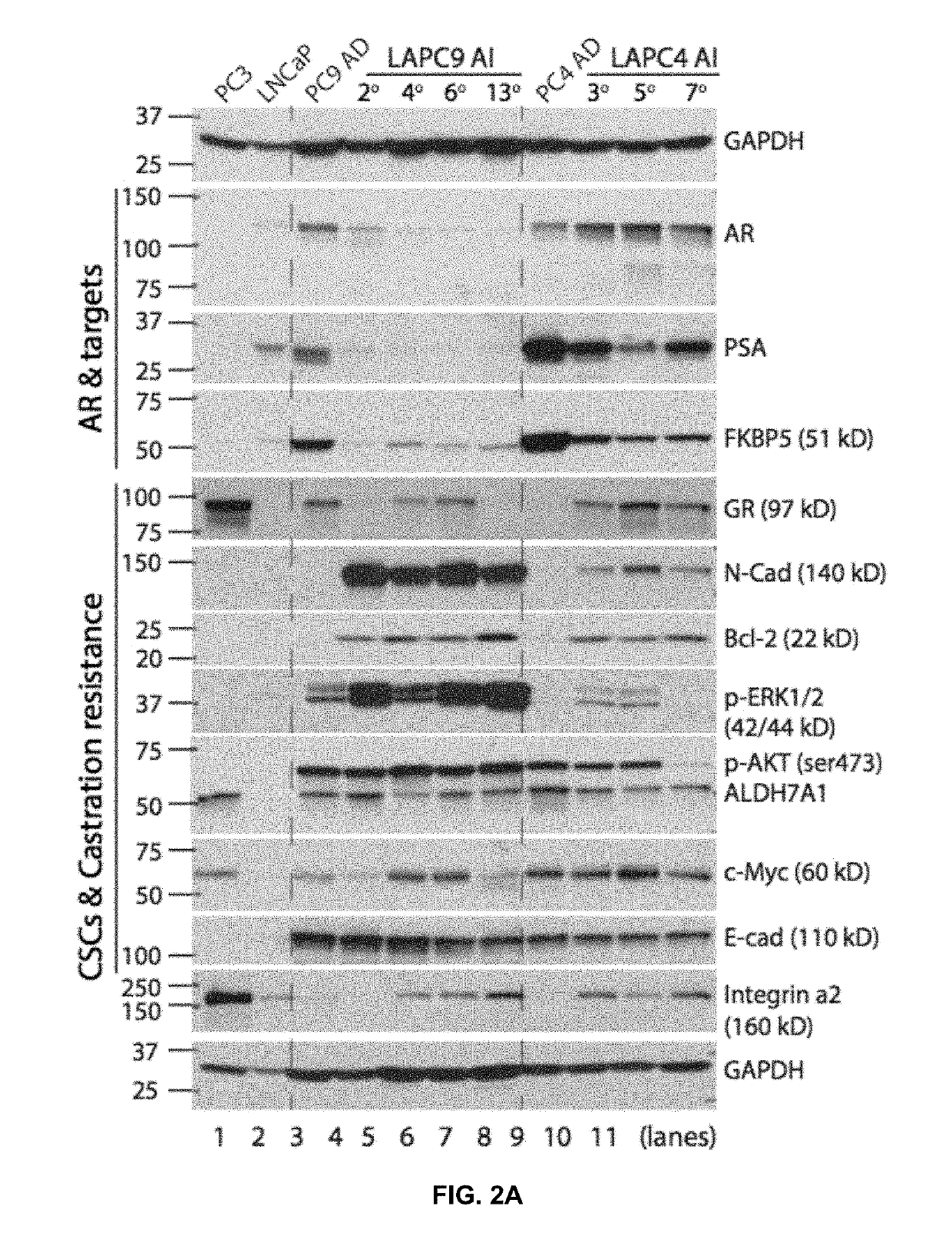
[0049]To determine the sensitivity of LAPC9 androgen-independent tumors to enzalutamide, therapy experiments were performed as described for the LNCaP primary AI tumors (FIG. 3). FIG. 4 shows that in sharp contrast to the observed reductions in tumor volume following Enzalutamide therapy, LNCaP primary androgen-independent tumors were refractory to enzalutamide administration (FIG. 4B). Western blotting (FIG. 4C) and immuno-histochemical (FIG. 4D) analysis revealed no expression of androgen receptors and PSA. FKBP5 levels were greatly diminished in both vehicle and treatment groups. On the other hand Enzalutamide treatment continued to express high levels of integrin α2, c-Myc, N-cadherin, Bcl-2, and p-ERK1 / 2 (FIG. 4C). To determine whether blocking alternate pathways regulated by any of these high expressing proteins, in the Enzalutamide resistant LAPC9 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com