Immunogenic Compositions for Use in Pneumococcal Vaccines
a technology of compositions and compositions, applied in the field of immunogenic compositions for use in pneumococcal vaccines, can solve the problems of limiting the number of conjugates, adversely affecting the immunogenicity of any individual conjugate, so as to increase the proportion of responders and increase the opa titers of human subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
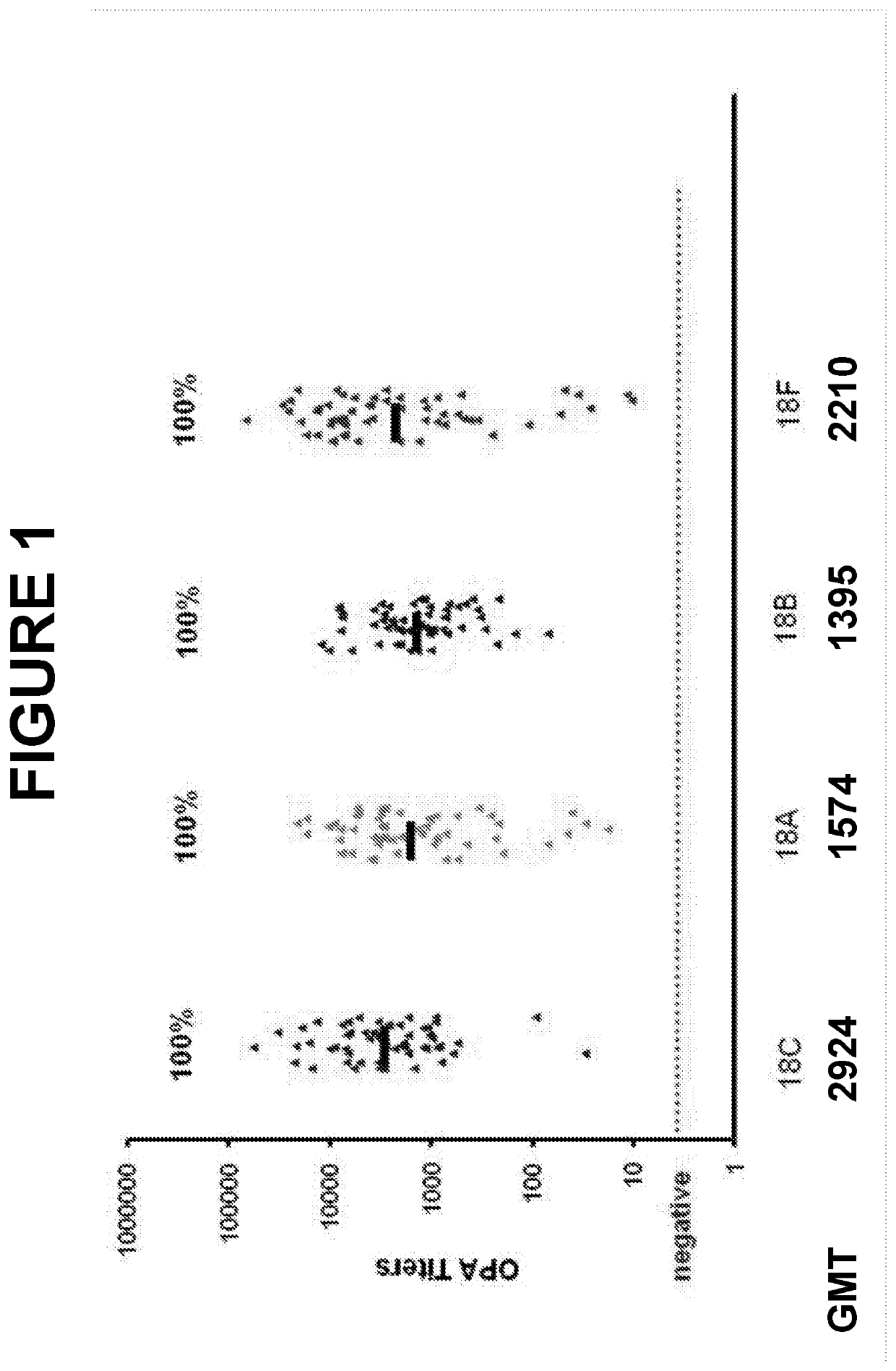
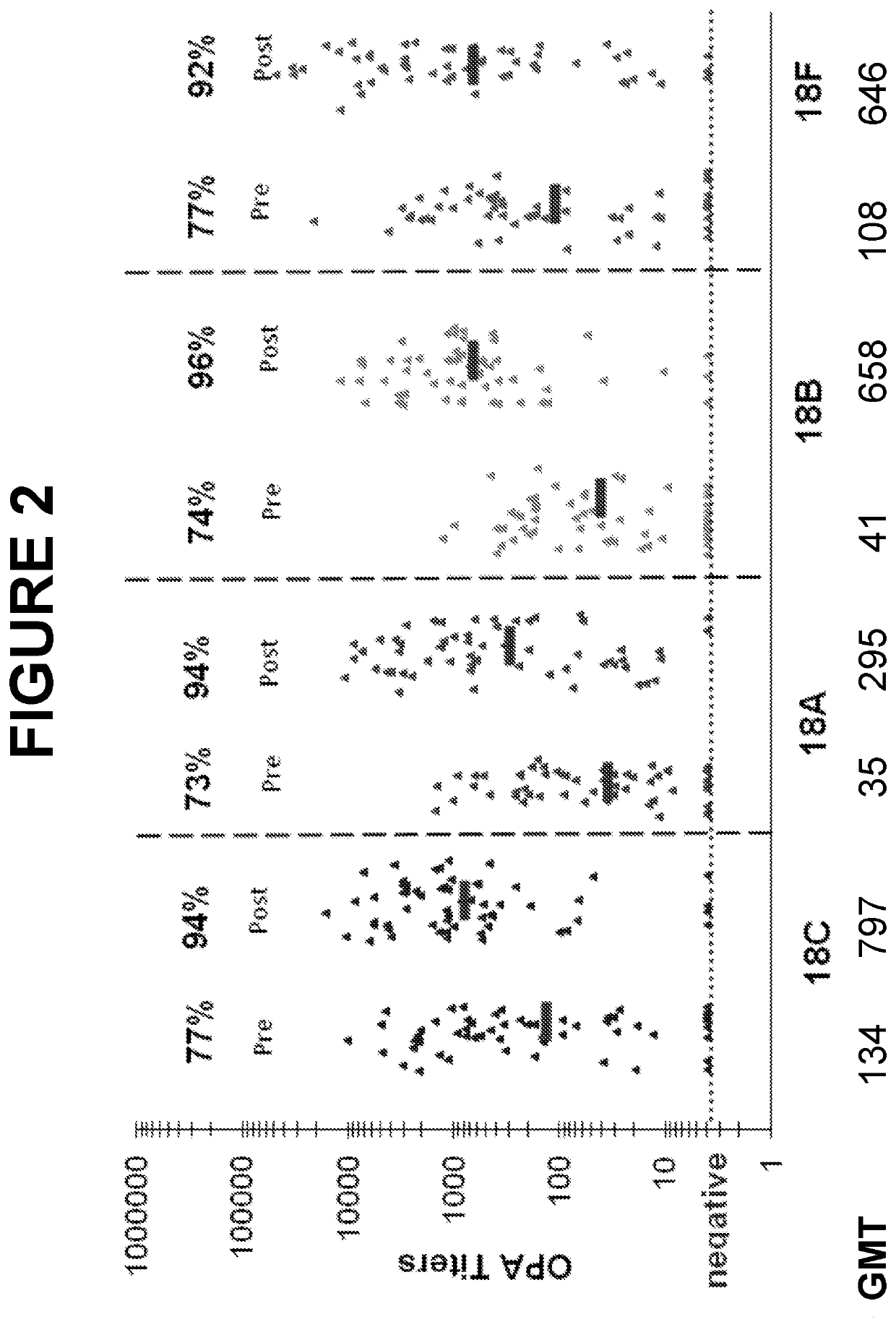
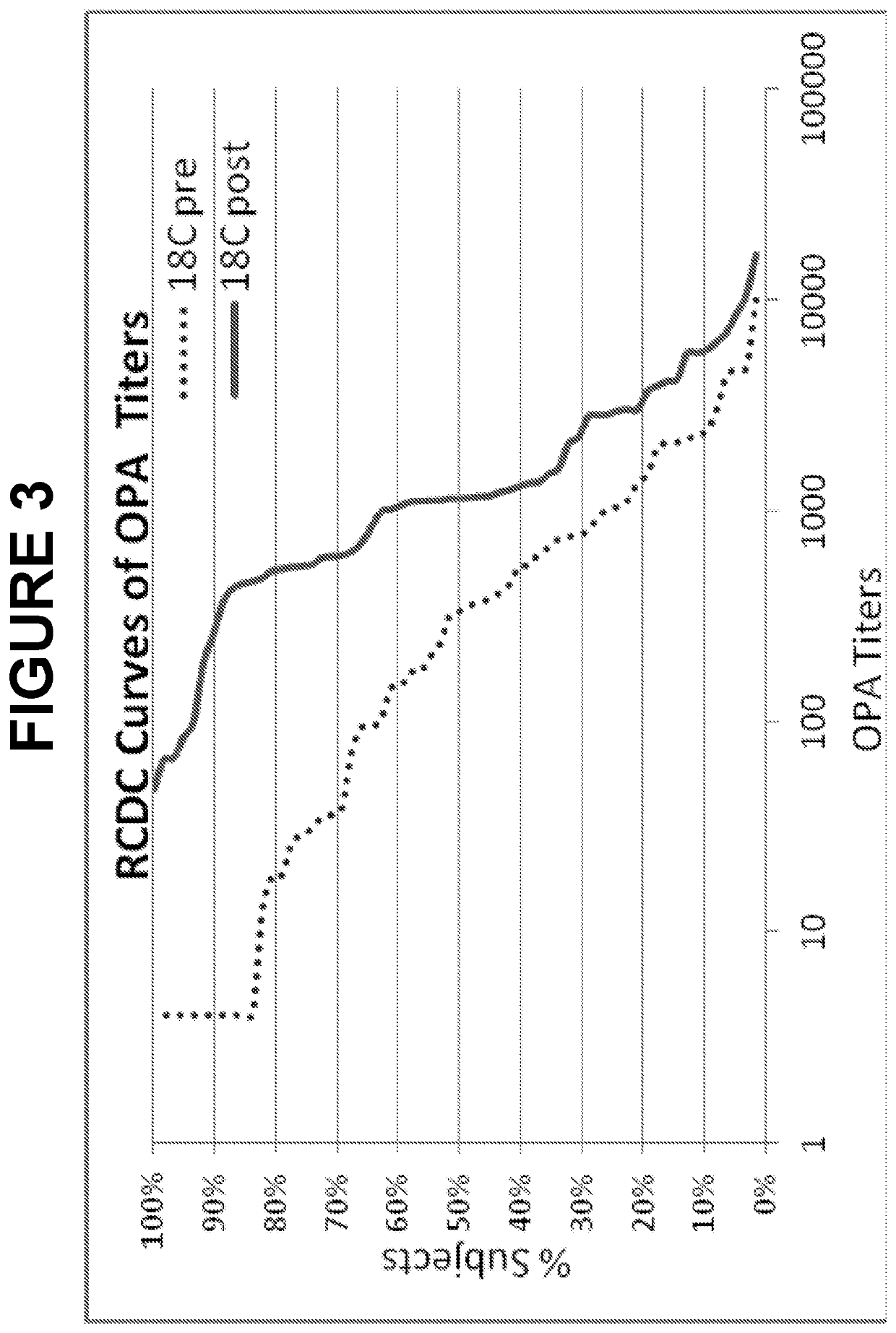
. Evaluation of Cross-Reactive Opsonophagocytic Immune Responses within Seroqroup 18 of Streptococcus pneumoniae
[0653]Host protection against S. pneumoniae is mediated primarily through anticapsular antibodydependent opsonophagocytosis. The pneumococcal opsonophagocytic assay (OPA), which measures killing of S. pneumoniae cells by phagocytic effector cells in the presence of functional antibody and complement, is considered to be an important surrogate for evaluating the effectiveness of pneumococcal vaccines.
Materials and Methods
Sera
[0654]Two randomly selected subsets of immune sera from adults vaccinated with a 13-valent pneumococcal conjugate vaccine (13vPnC) were tested in OPA assays for the serotypes 18F, 18A, 18B and 18C. The sera were collected from U.S. clinical trials 6115A1-004 (N=59, post-vaccinated) and 6115A1-3005 (N=66, matched pre-and post-vaccination), respectively.
[0655]Study 6115A1-3005 (ClinicalTrials.gov Identifier: NCT00546572) was a phase 3, randomized, active-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com