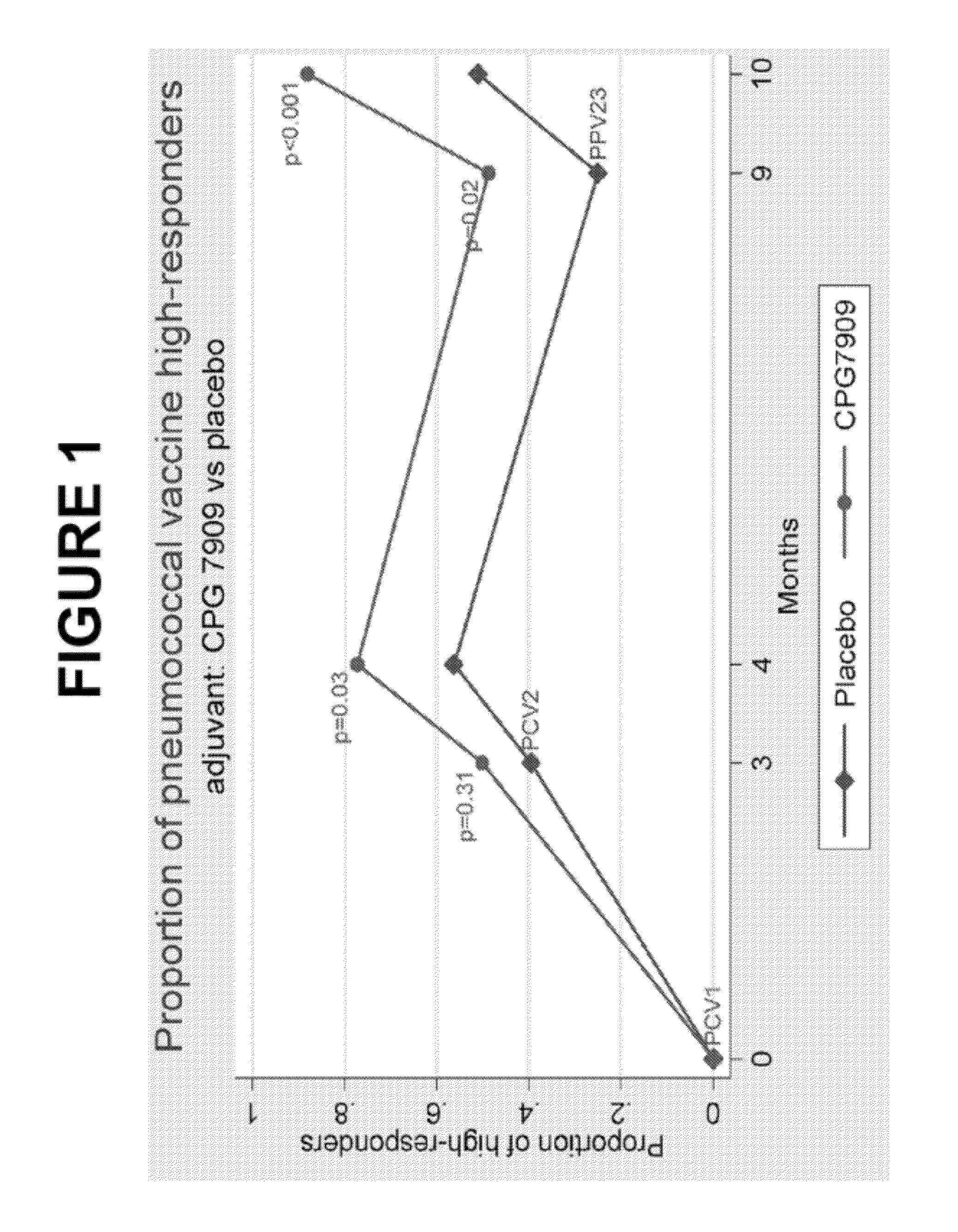
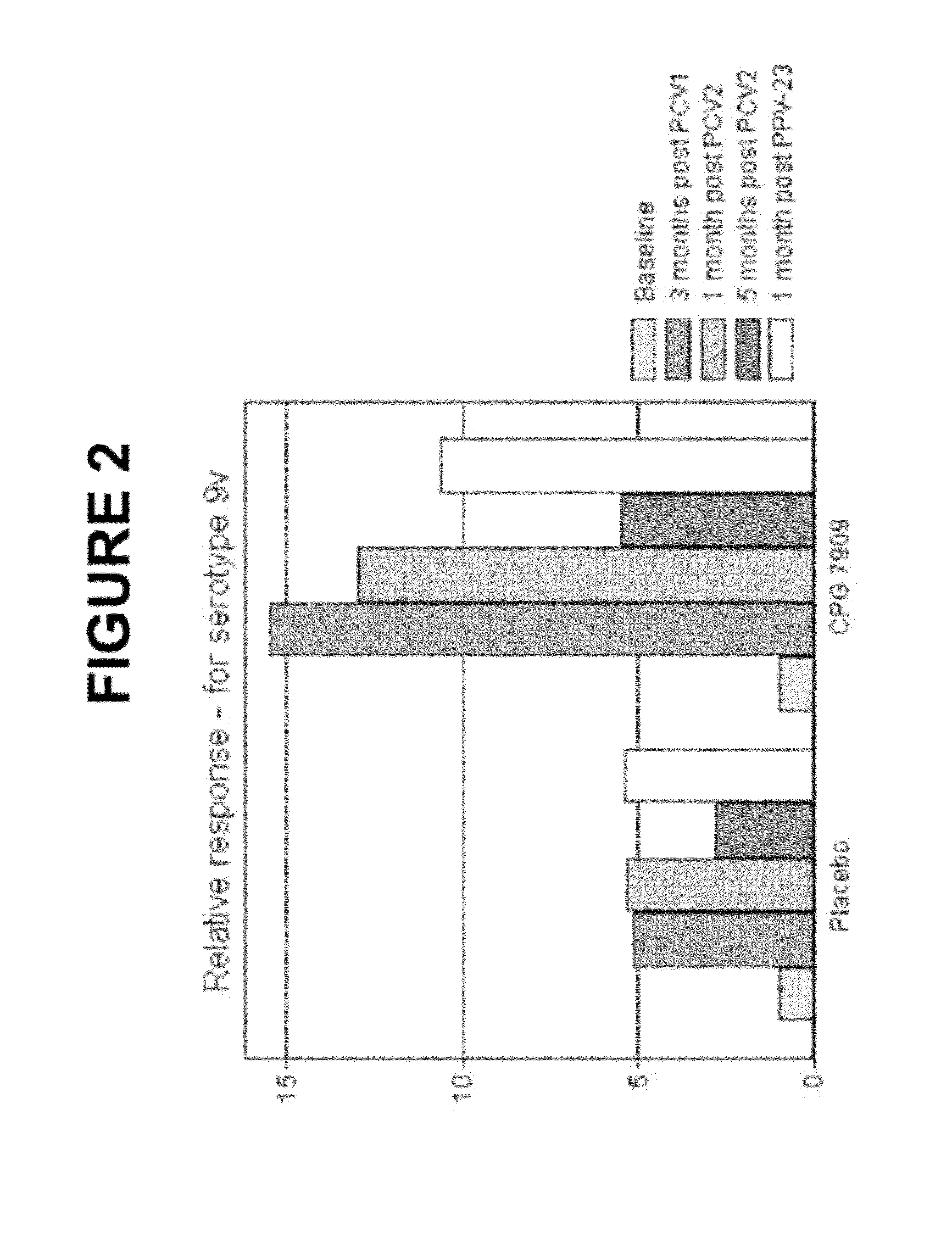
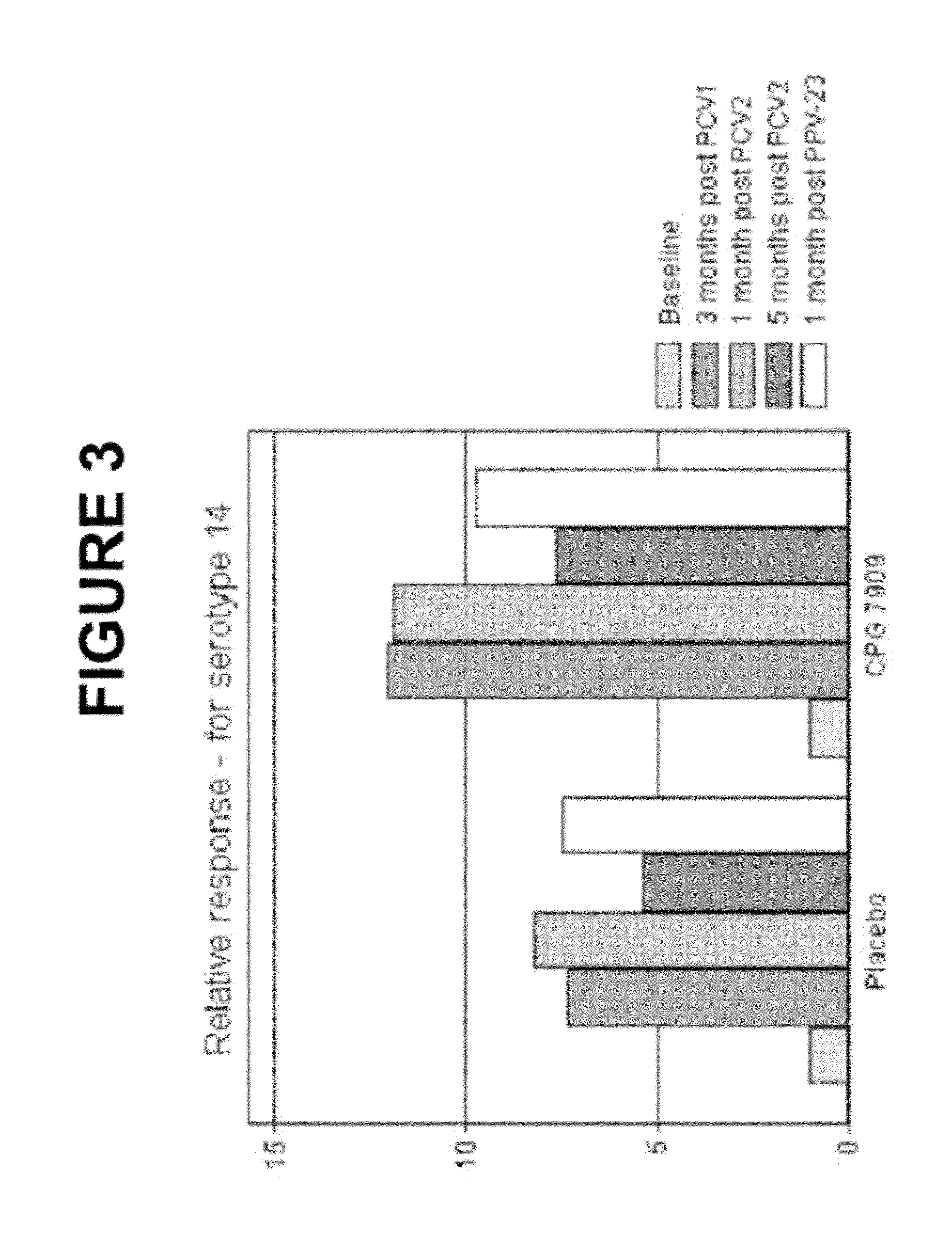
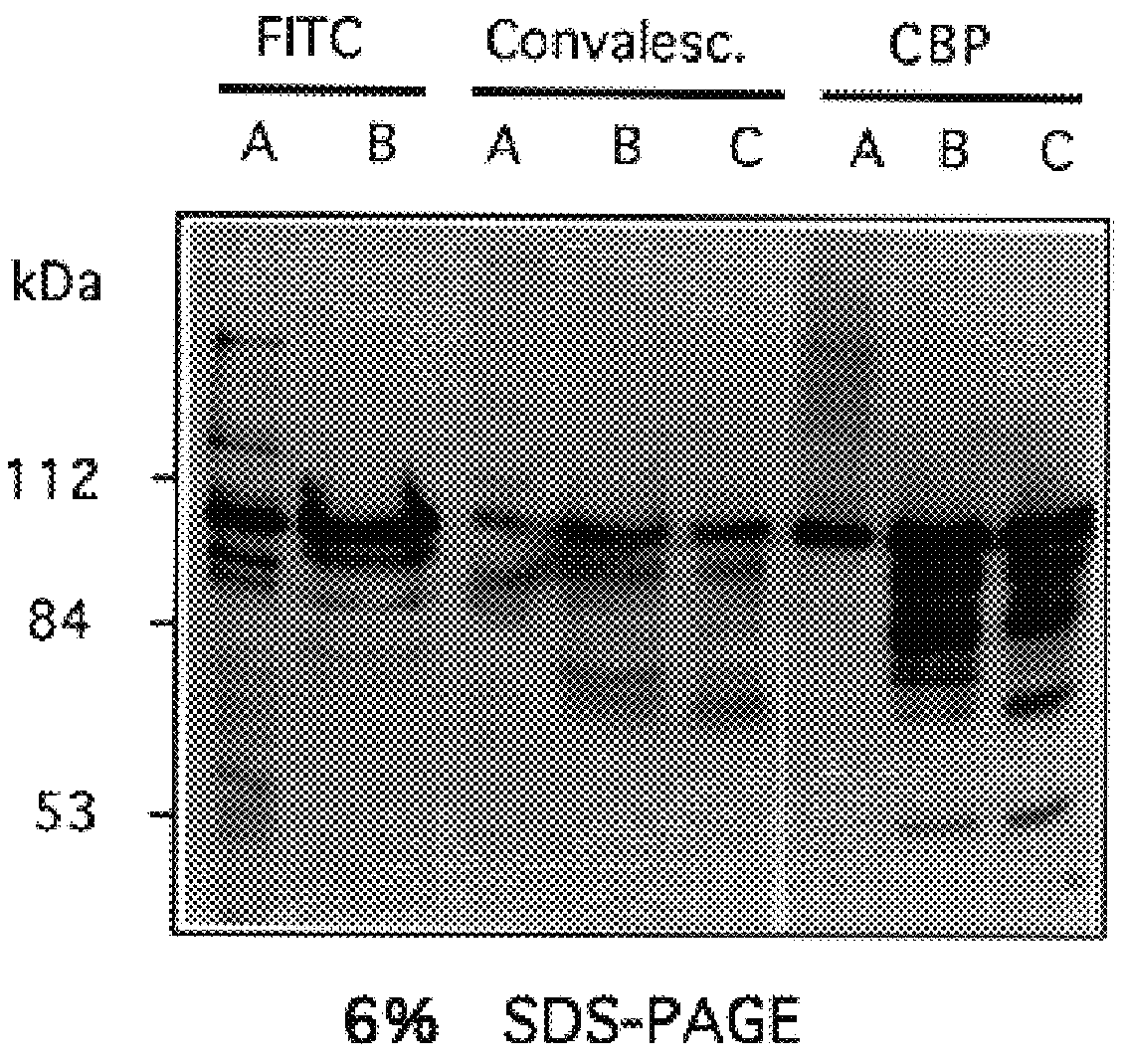
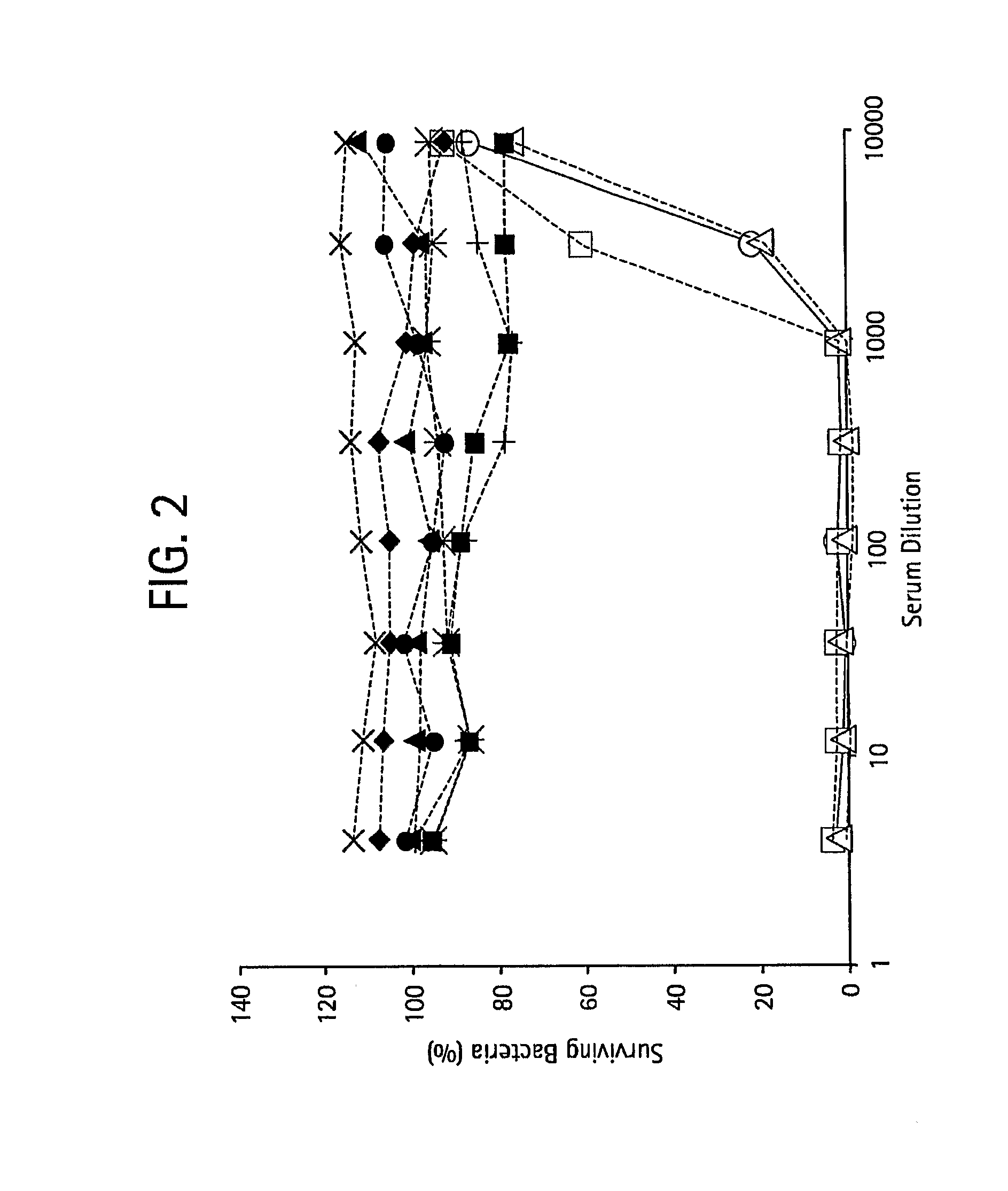
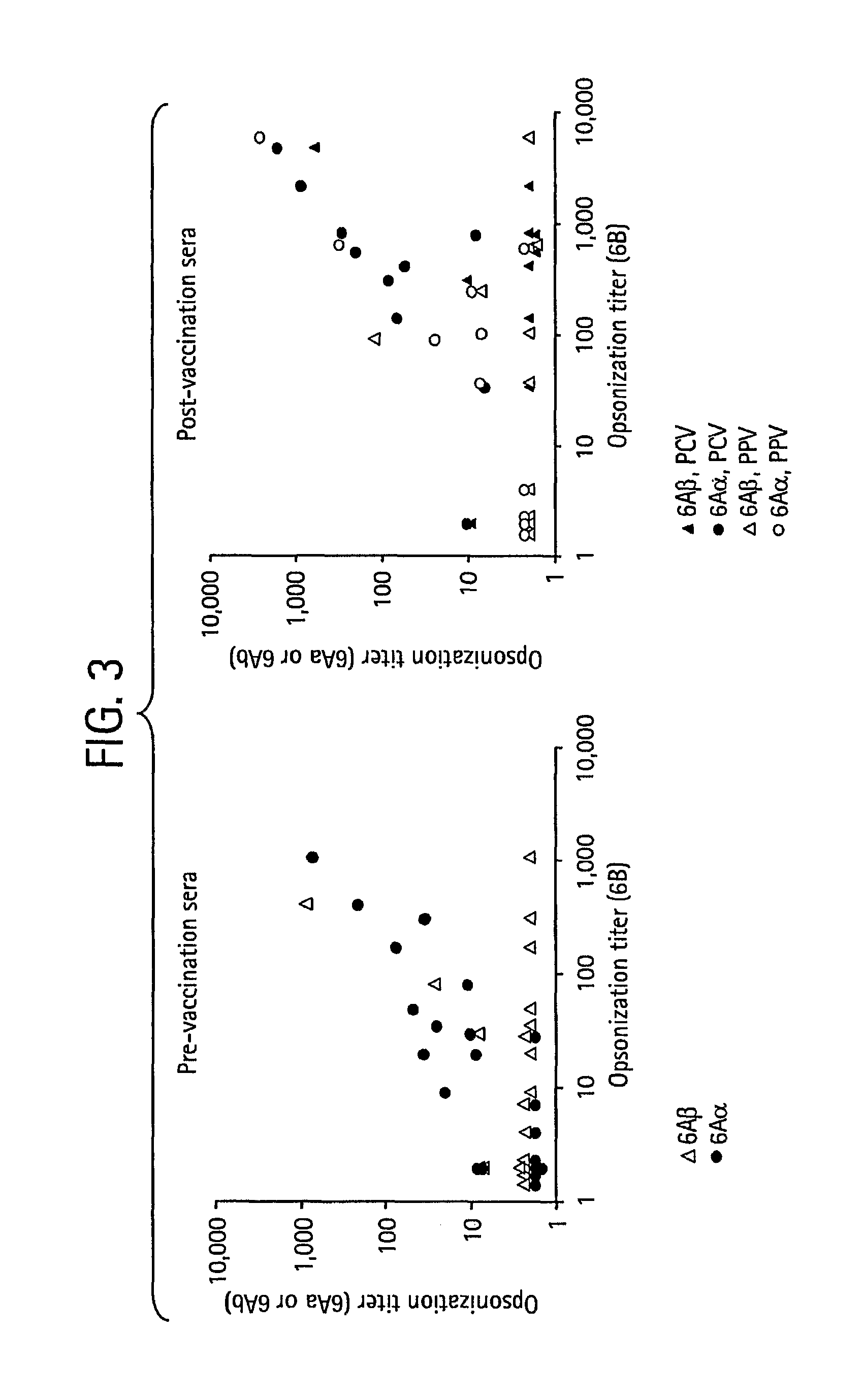
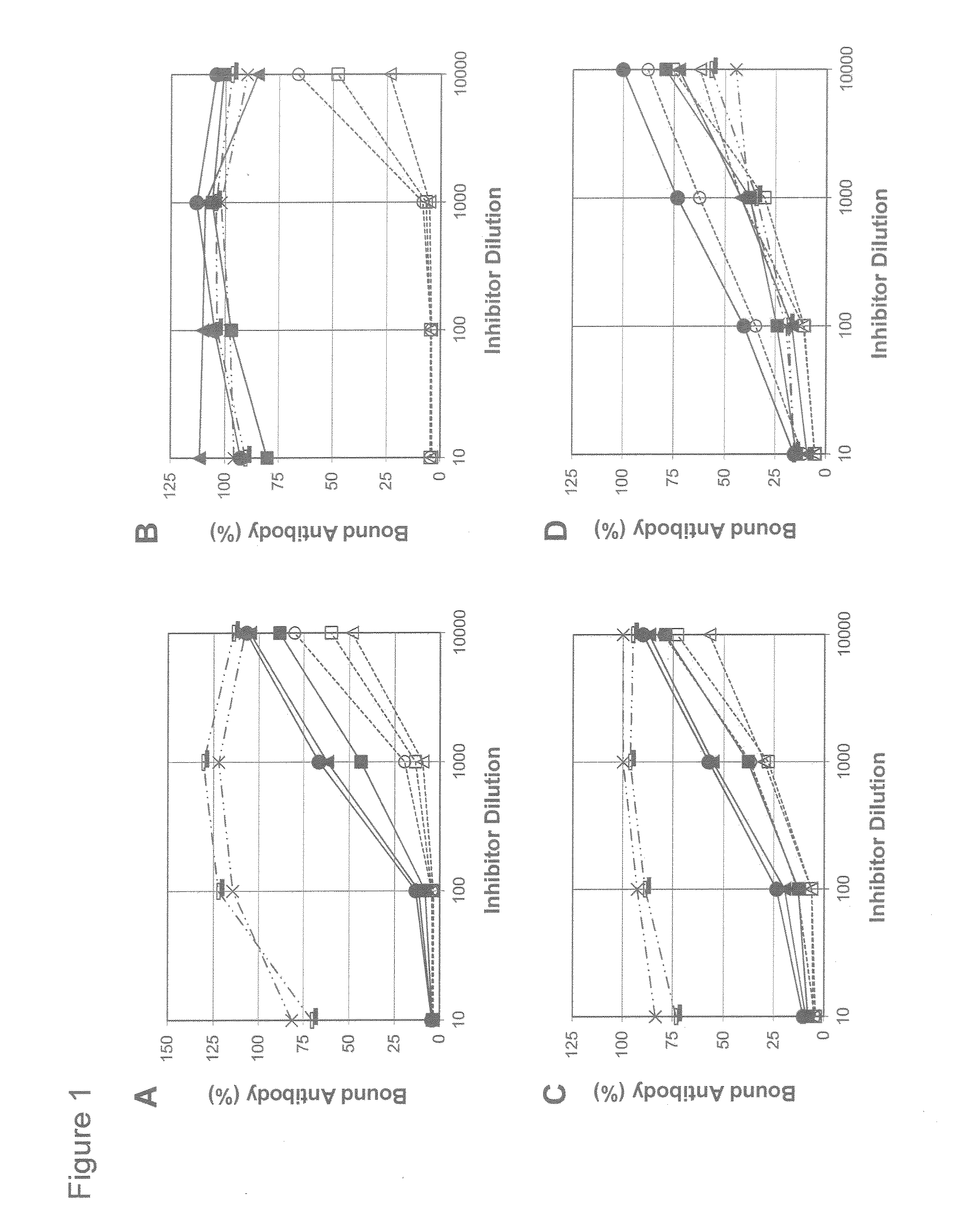
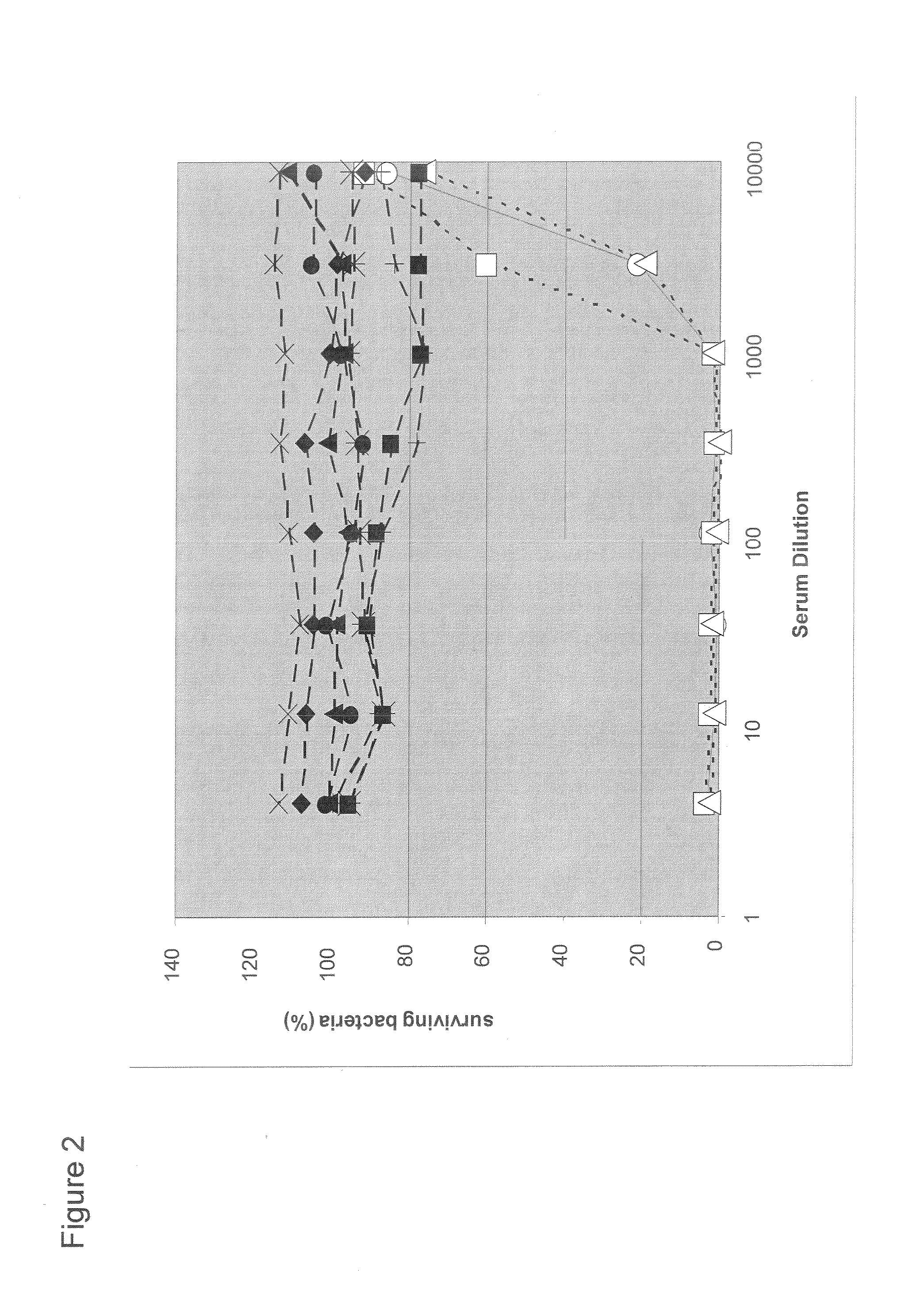
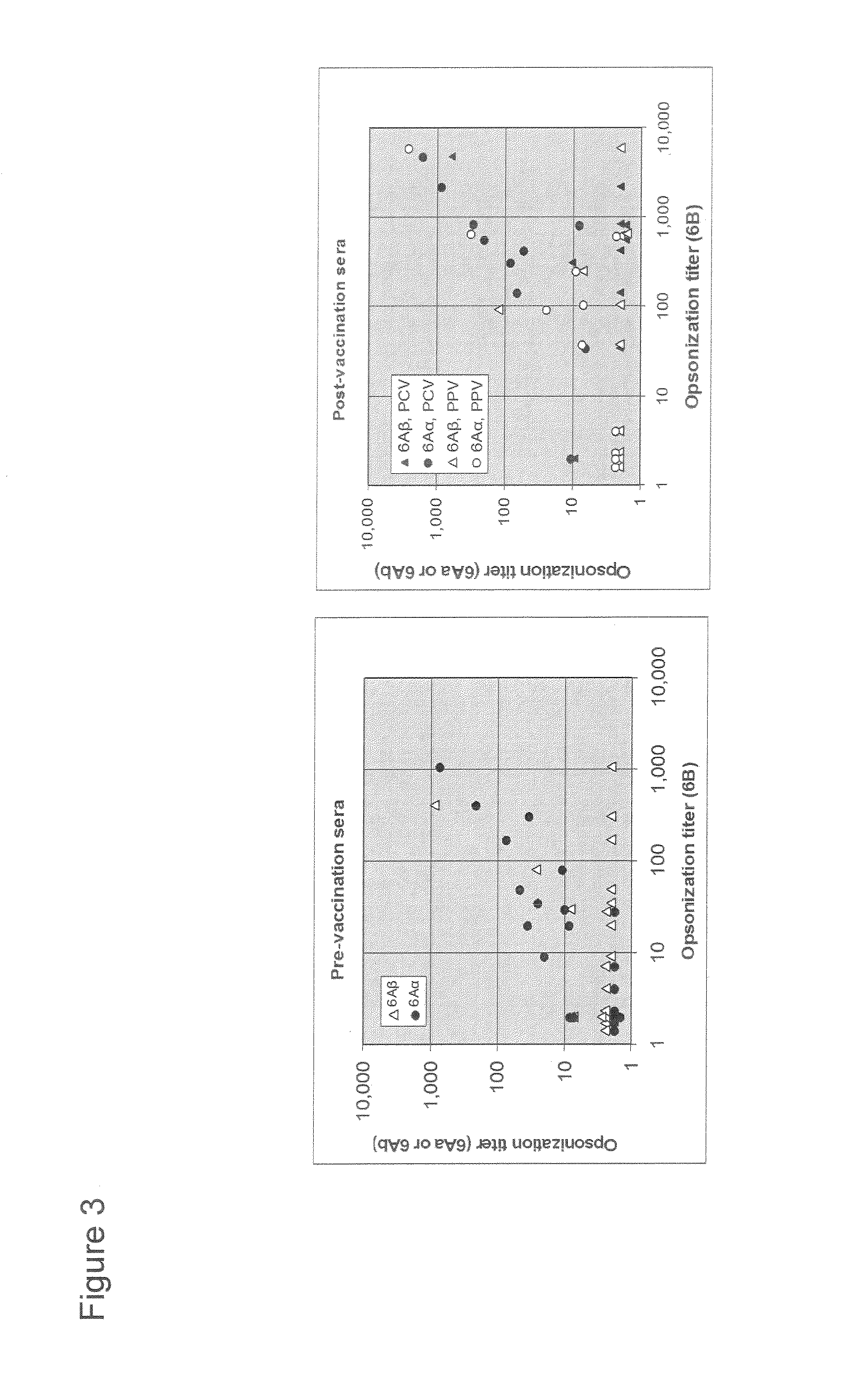
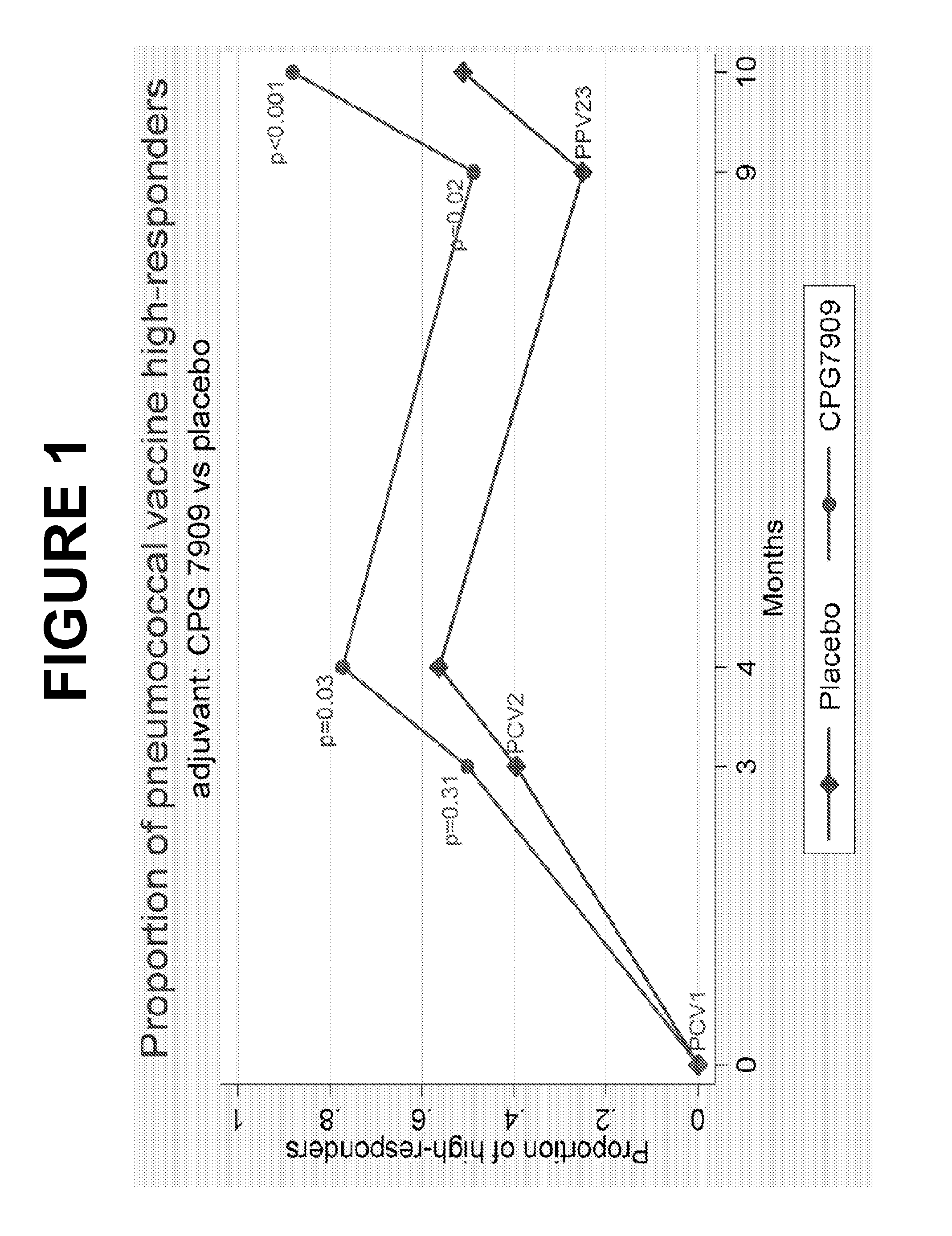
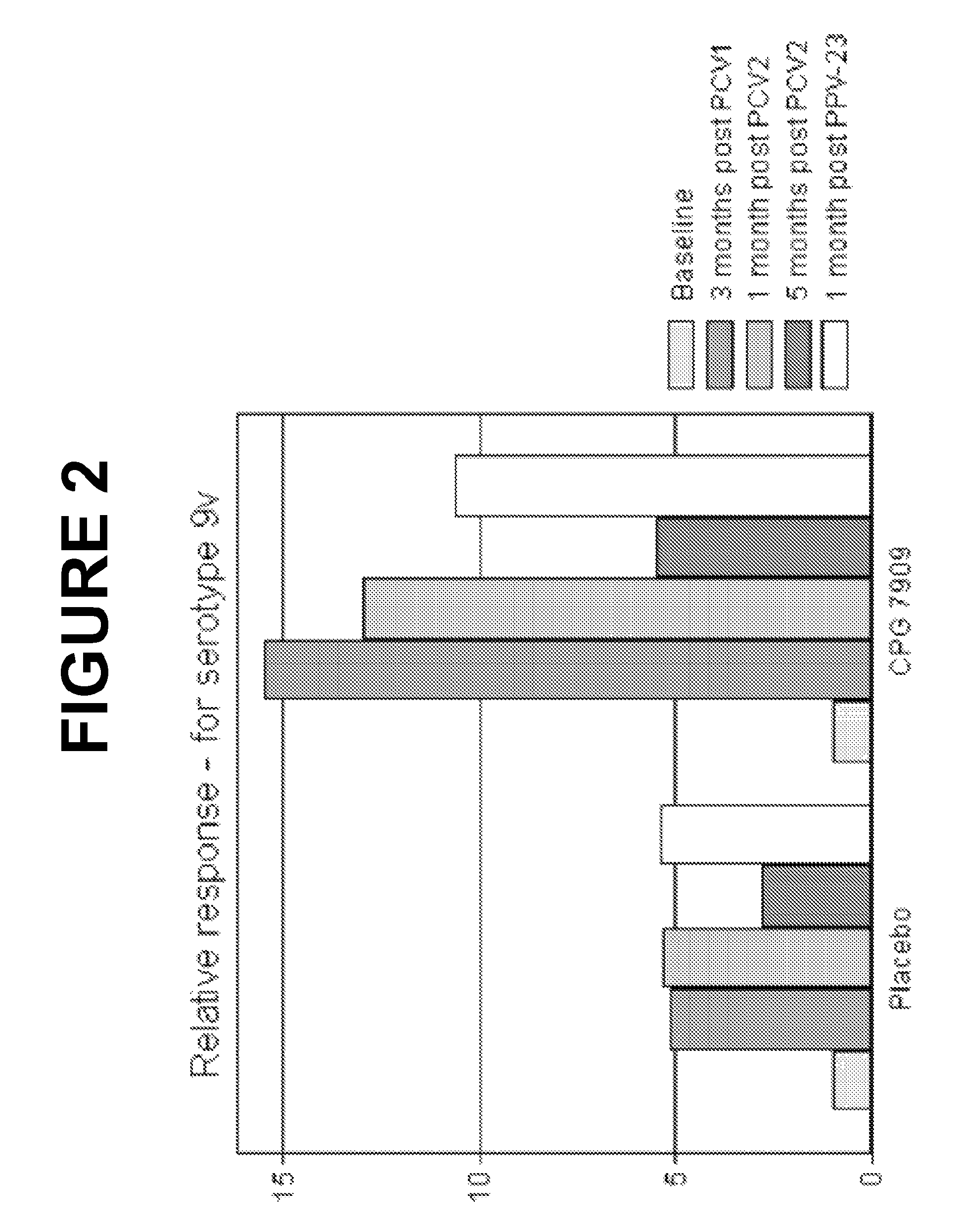
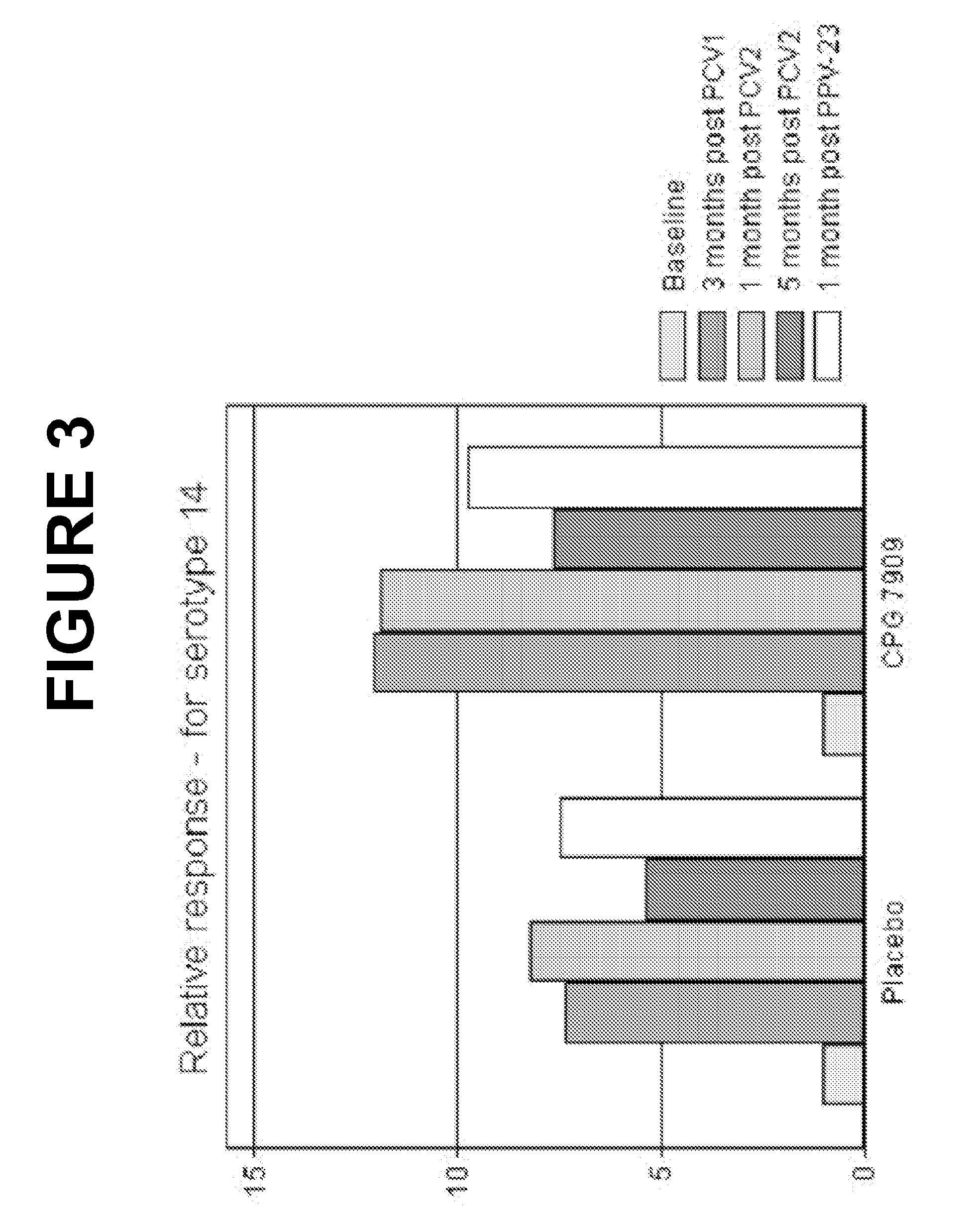
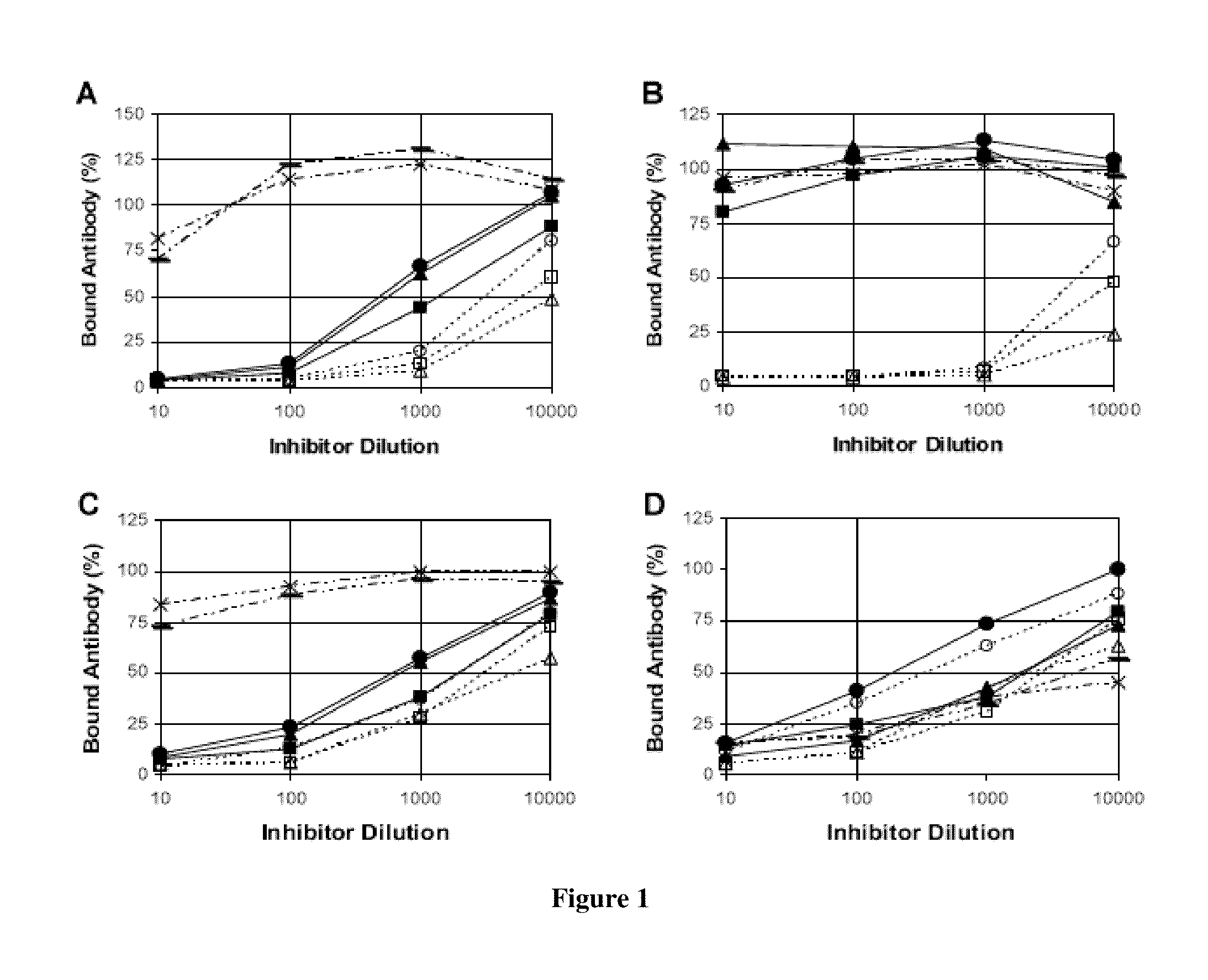
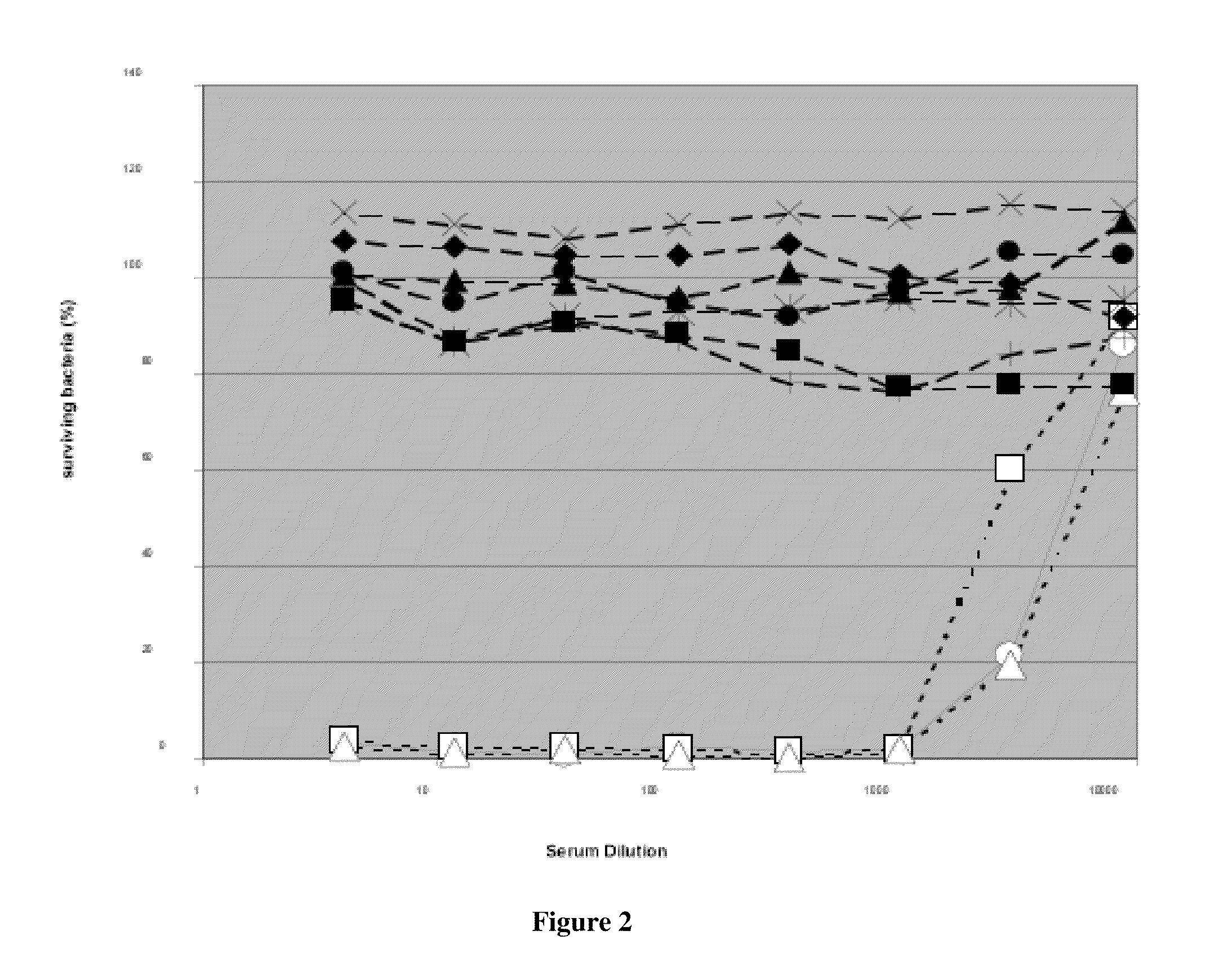
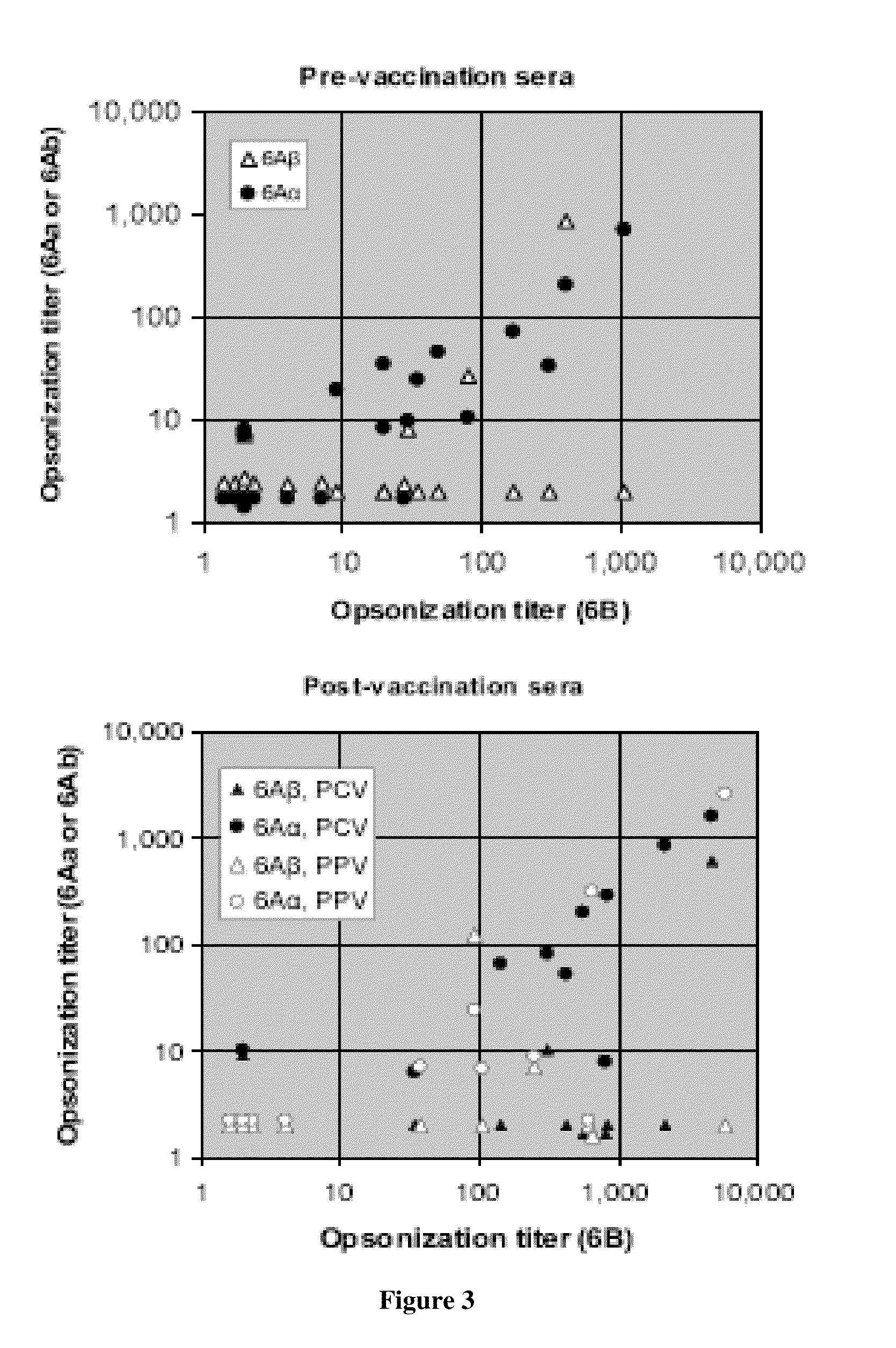
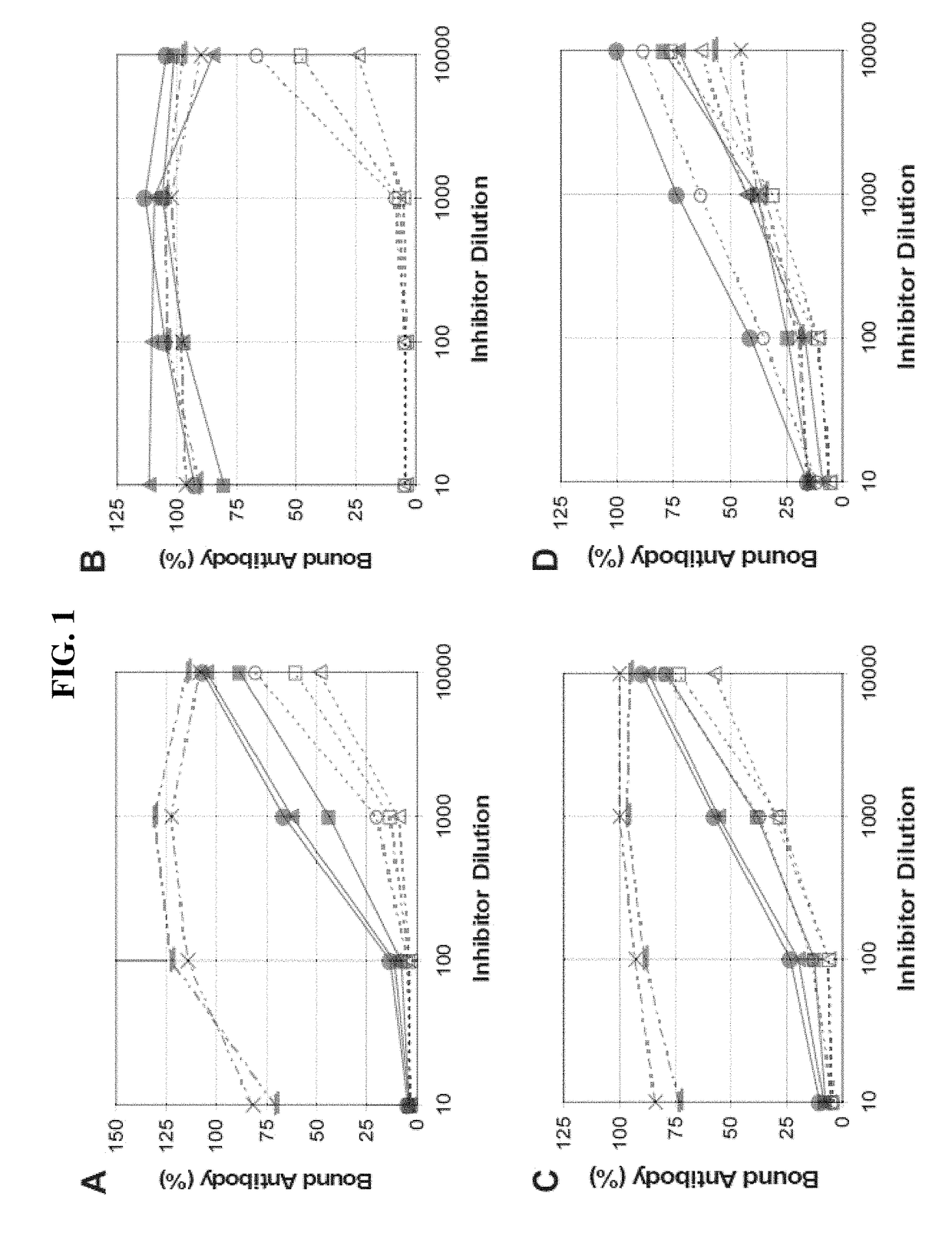
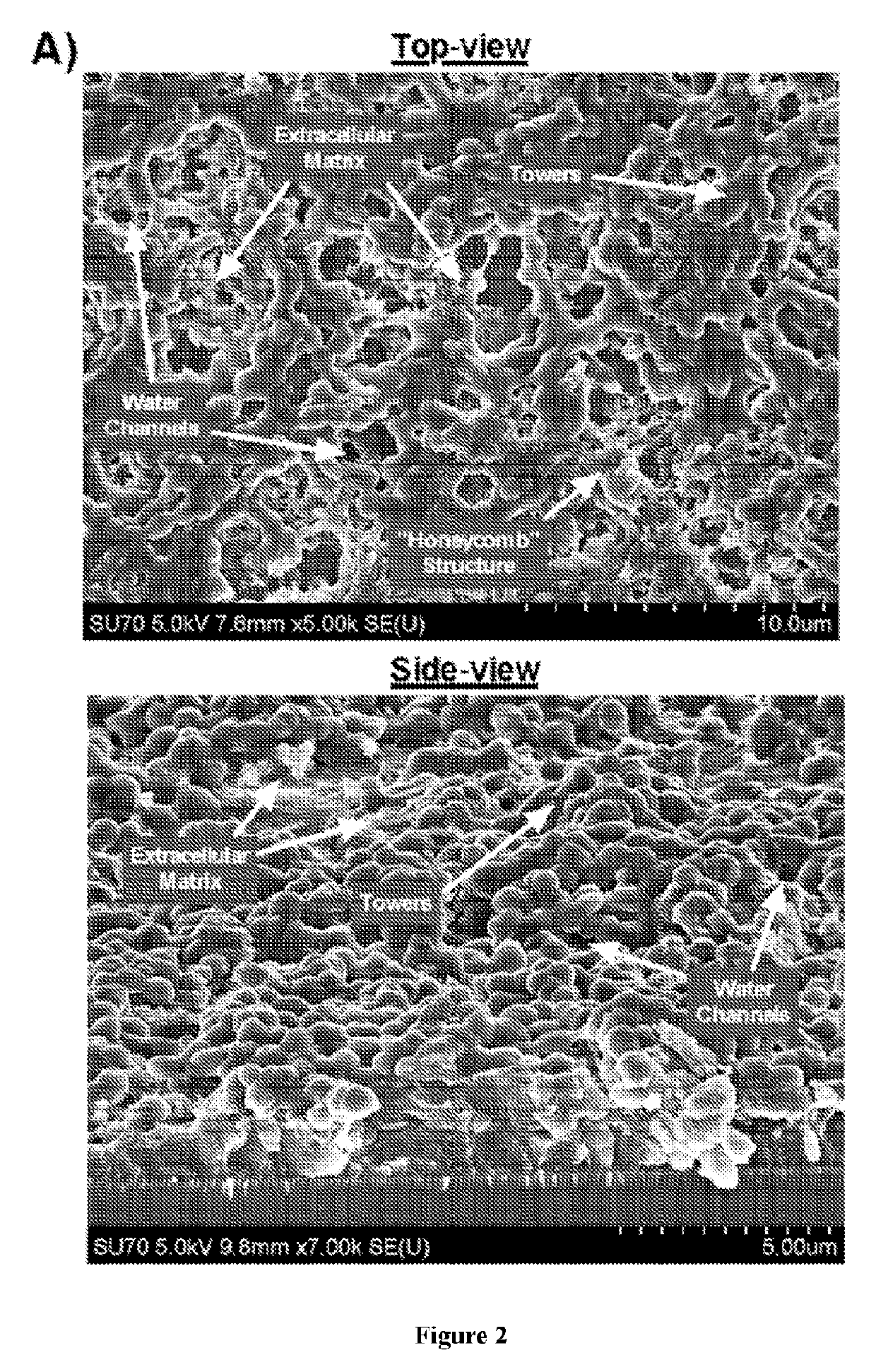
Patents
Literature
30 results about "Pneumococcal vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pneumococcal vaccines are vaccines against the bacterium Streptococcus pneumoniae. Their use can prevent some cases of pneumonia, meningitis, and sepsis. There are two types of pneumococcal vaccines: conjugate vaccines and polysaccharide vaccines. They are given by injection either into a muscle or just under the skin.
Pneumococcal vaccine and uses thereof
InactiveUS20120052088A1Low toxicitySafe and effective T-cell dependent carrierAntibacterial agentsNervous disorderCoccidiaSurgery
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumococcal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARM GRP INC
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS6245335B1Improve efficiencySufficient quantityAntibacterial agentsBacteriaBacteroidesCholine binding protein
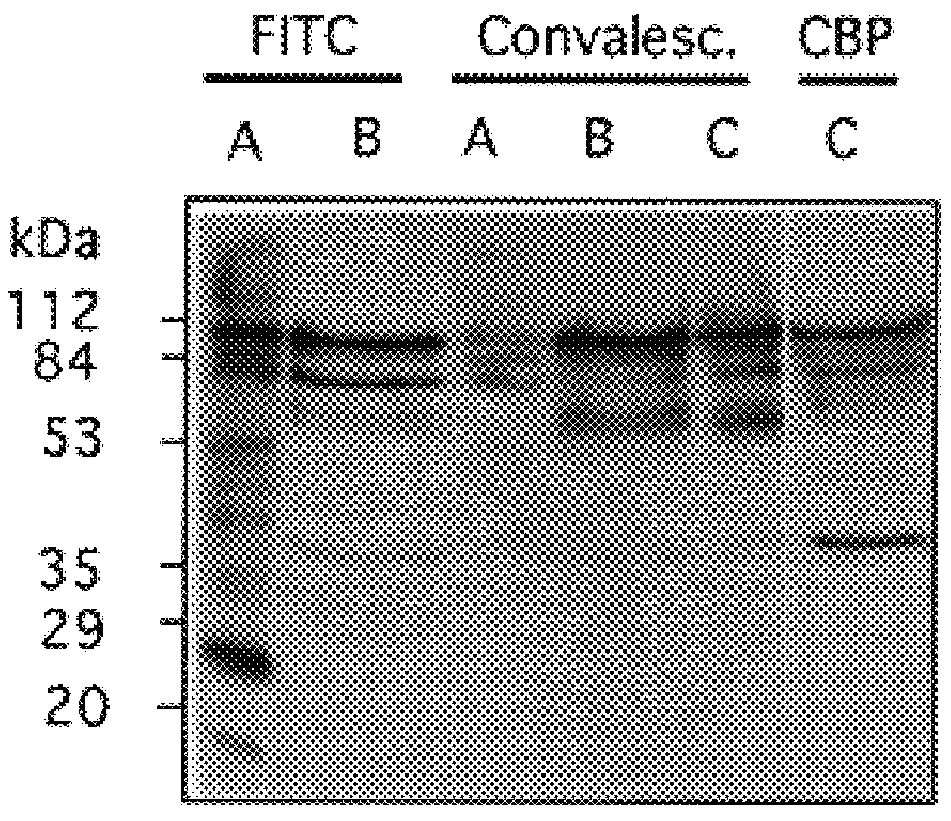
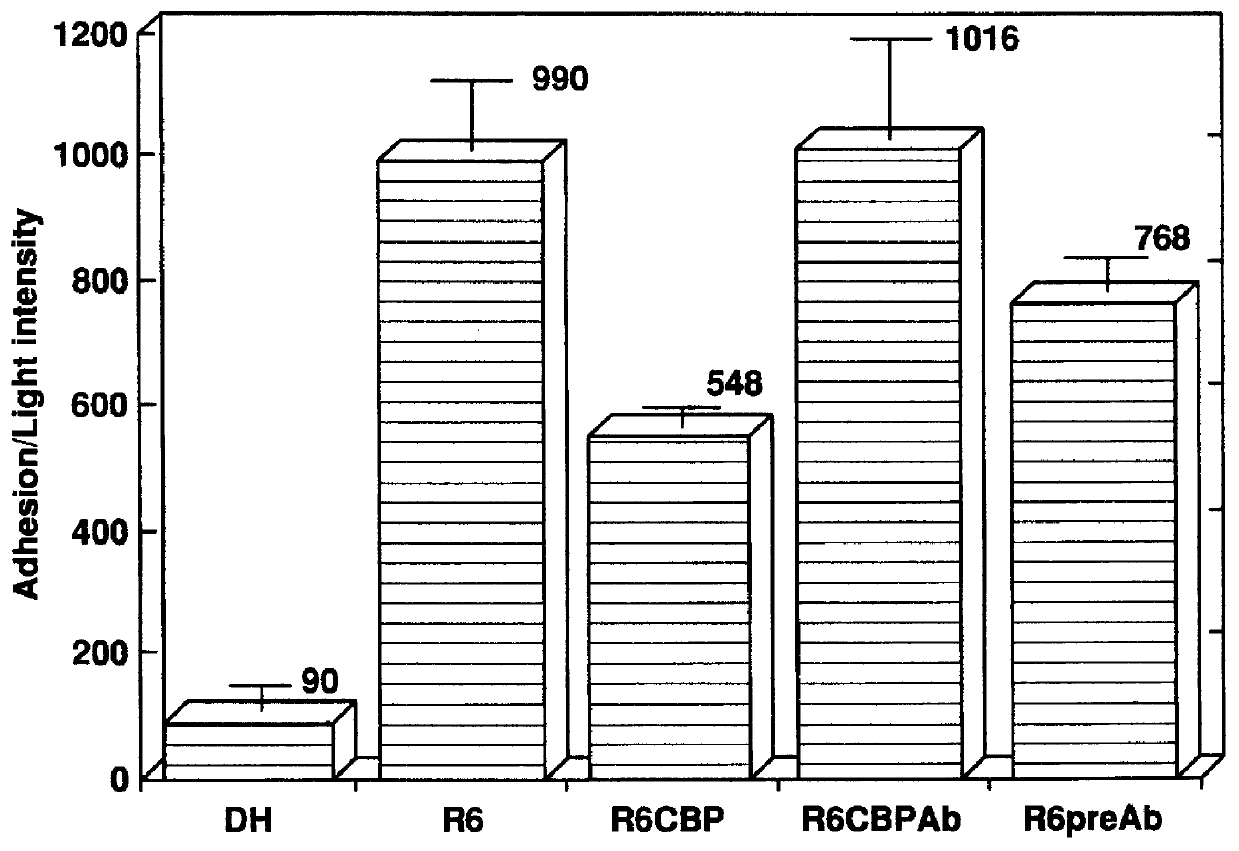
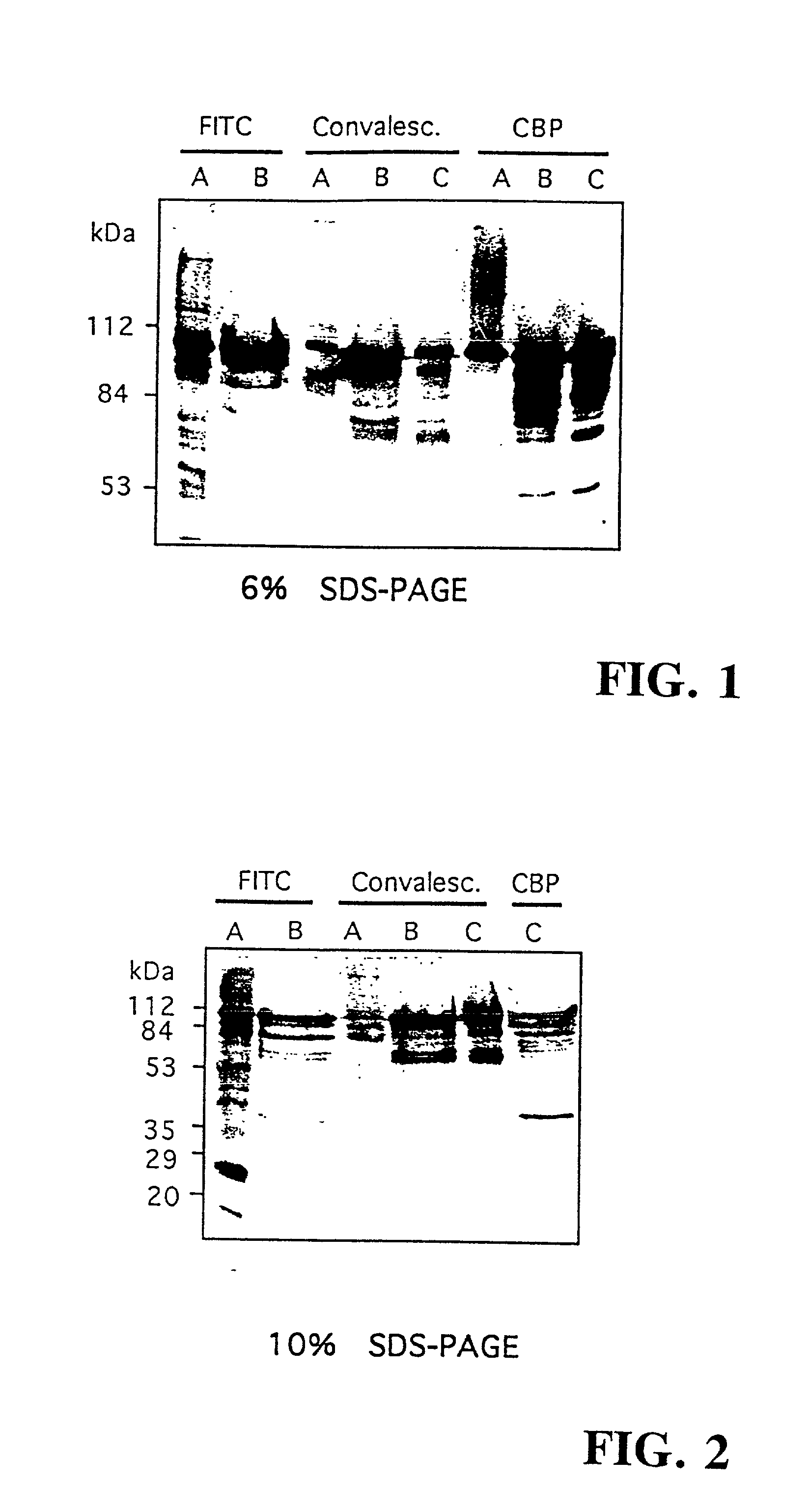
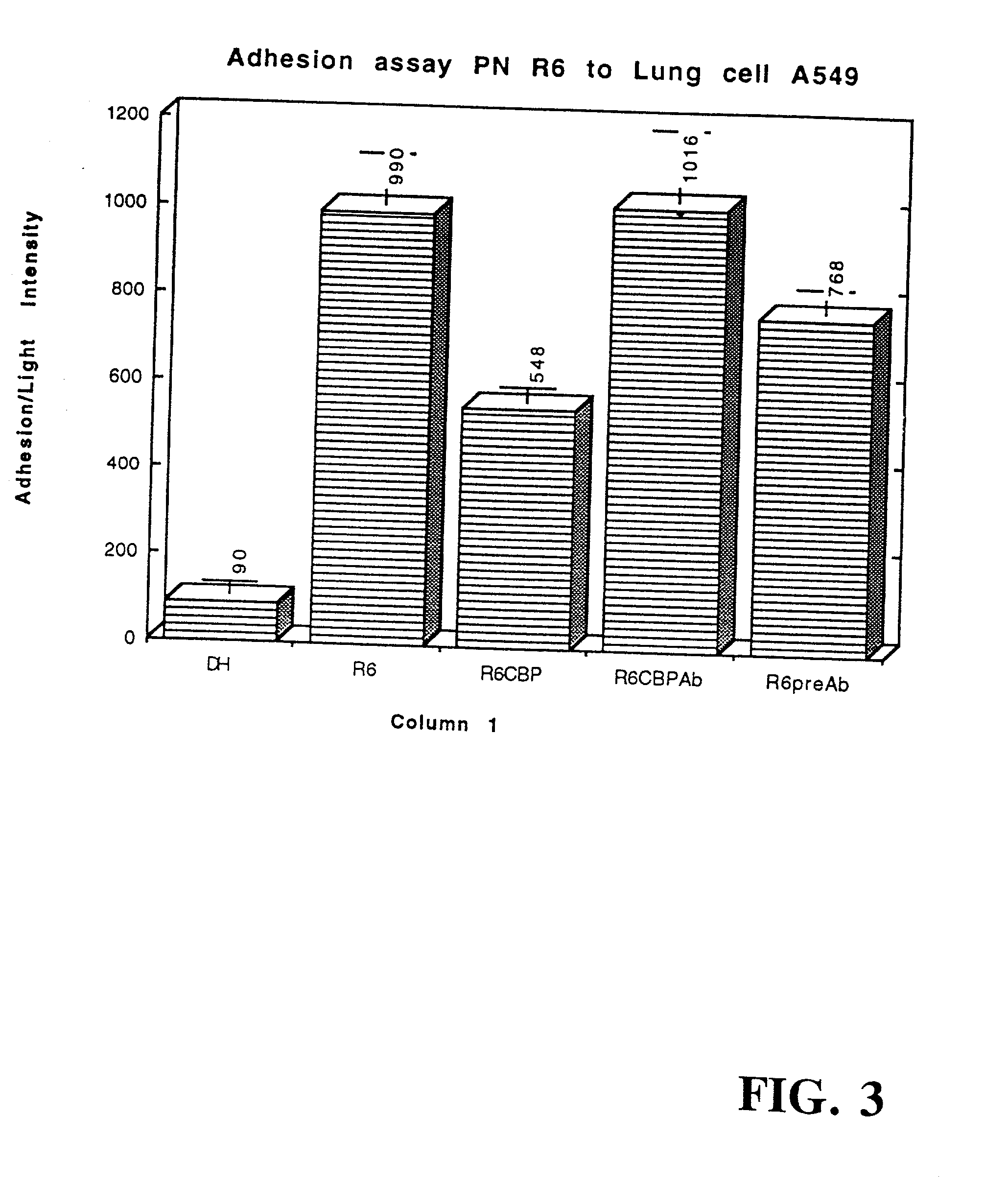
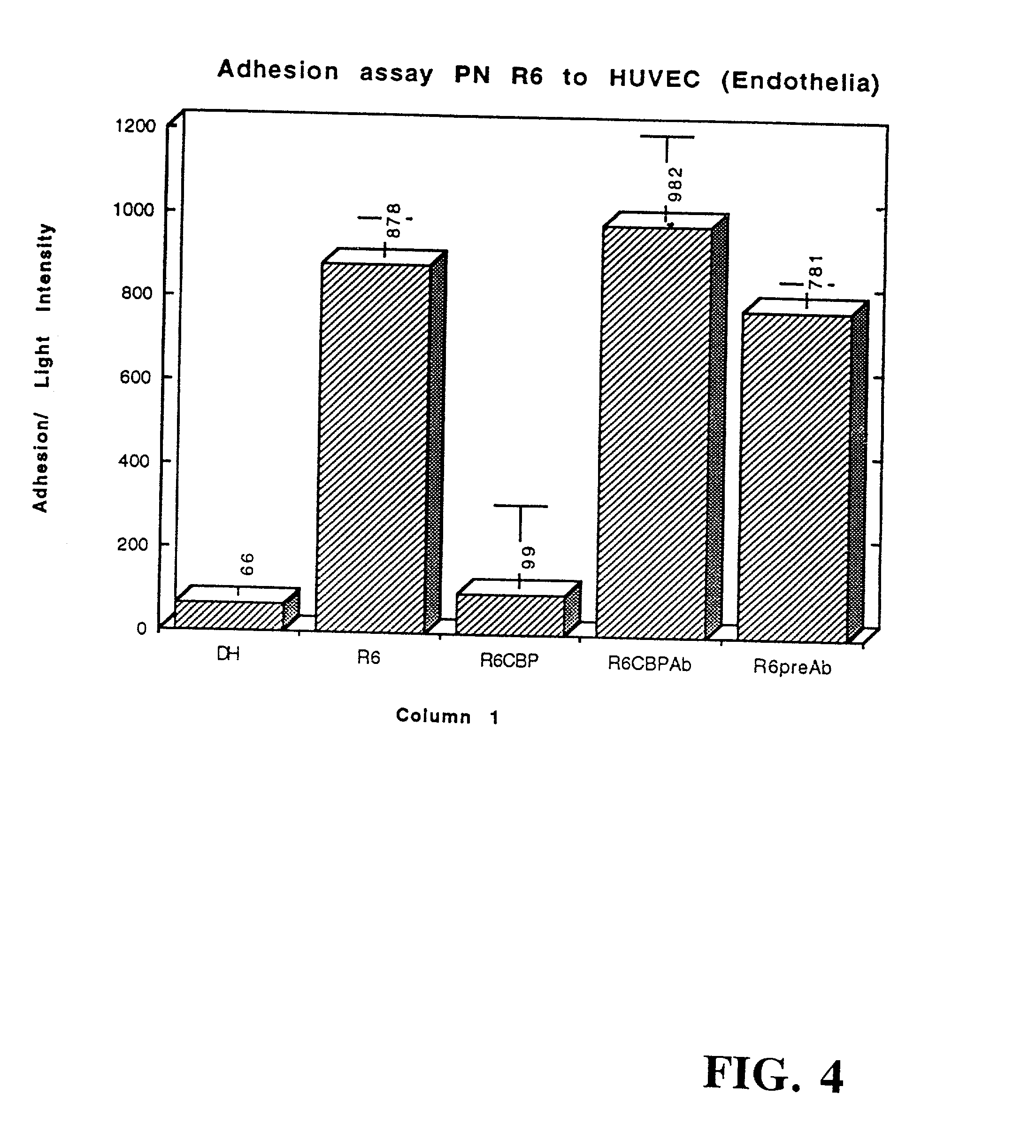
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
Pneumococcal Vaccine and Uses Thereof
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumoccocal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARMA GRP INC
Immunogenic Compositions for Use in Pneumococcal Vaccines
ActiveUS20180000922A1Raise the ratioIncrease OPA titerAntibacterial agentsBacterial antigen ingredientsPneumococcal vaccineImmunogenicity
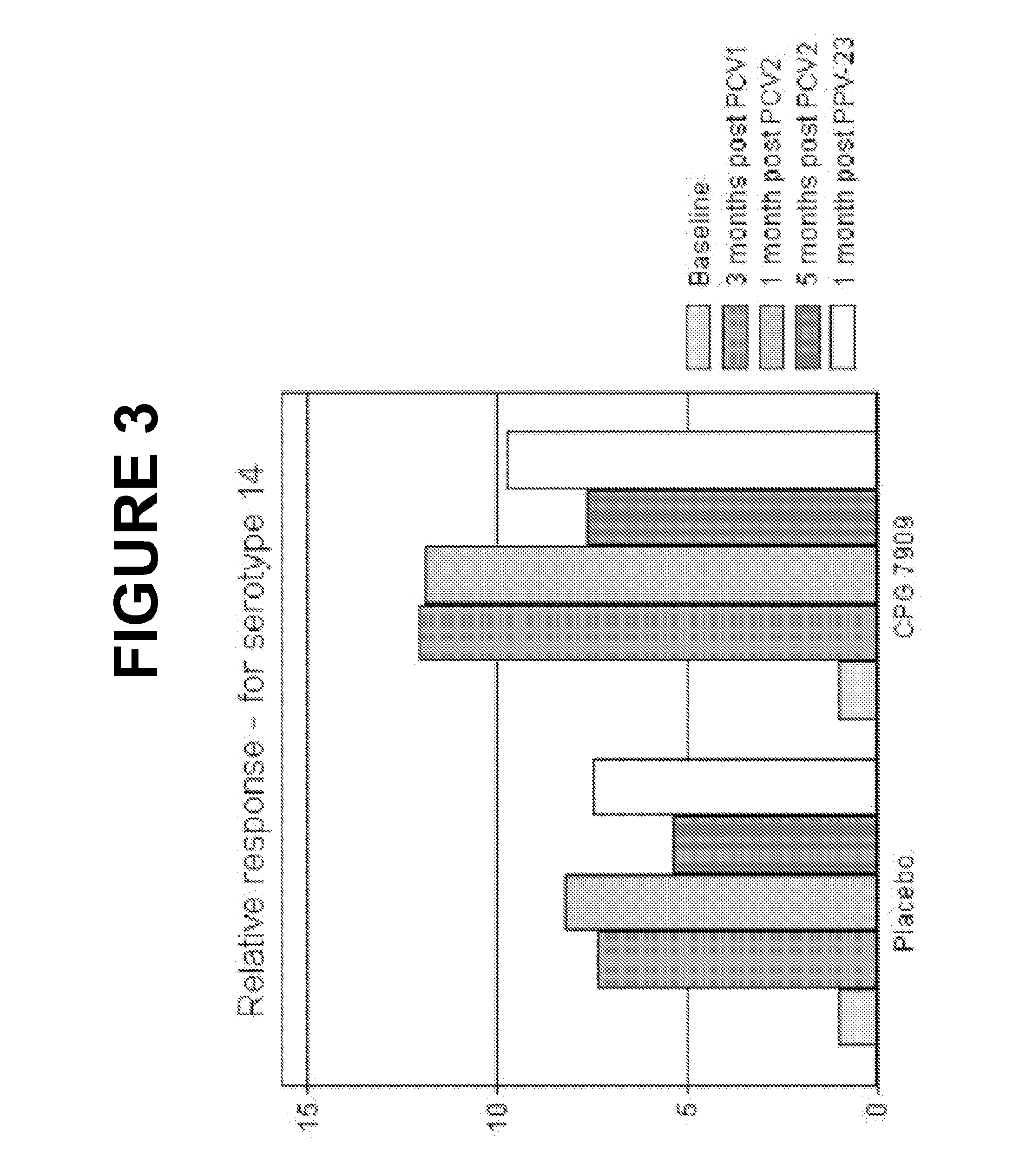
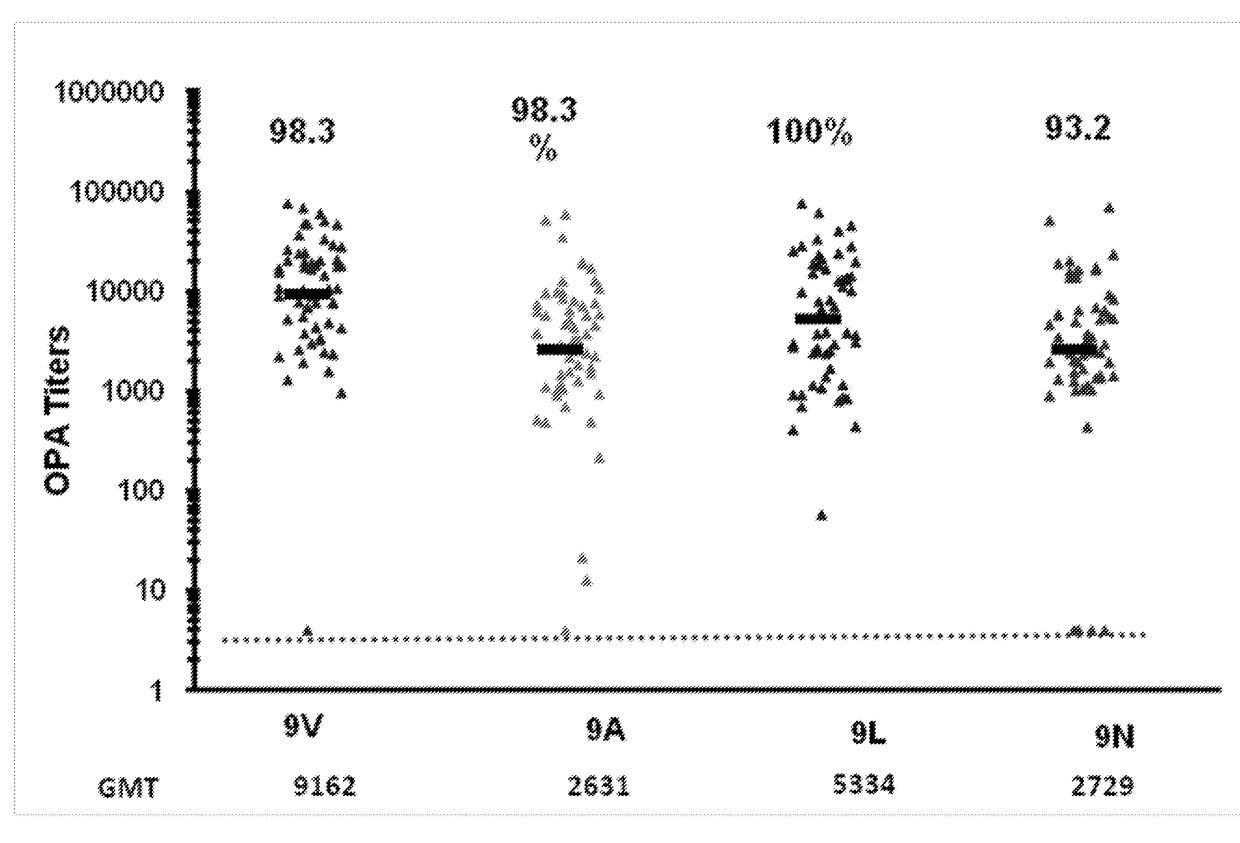
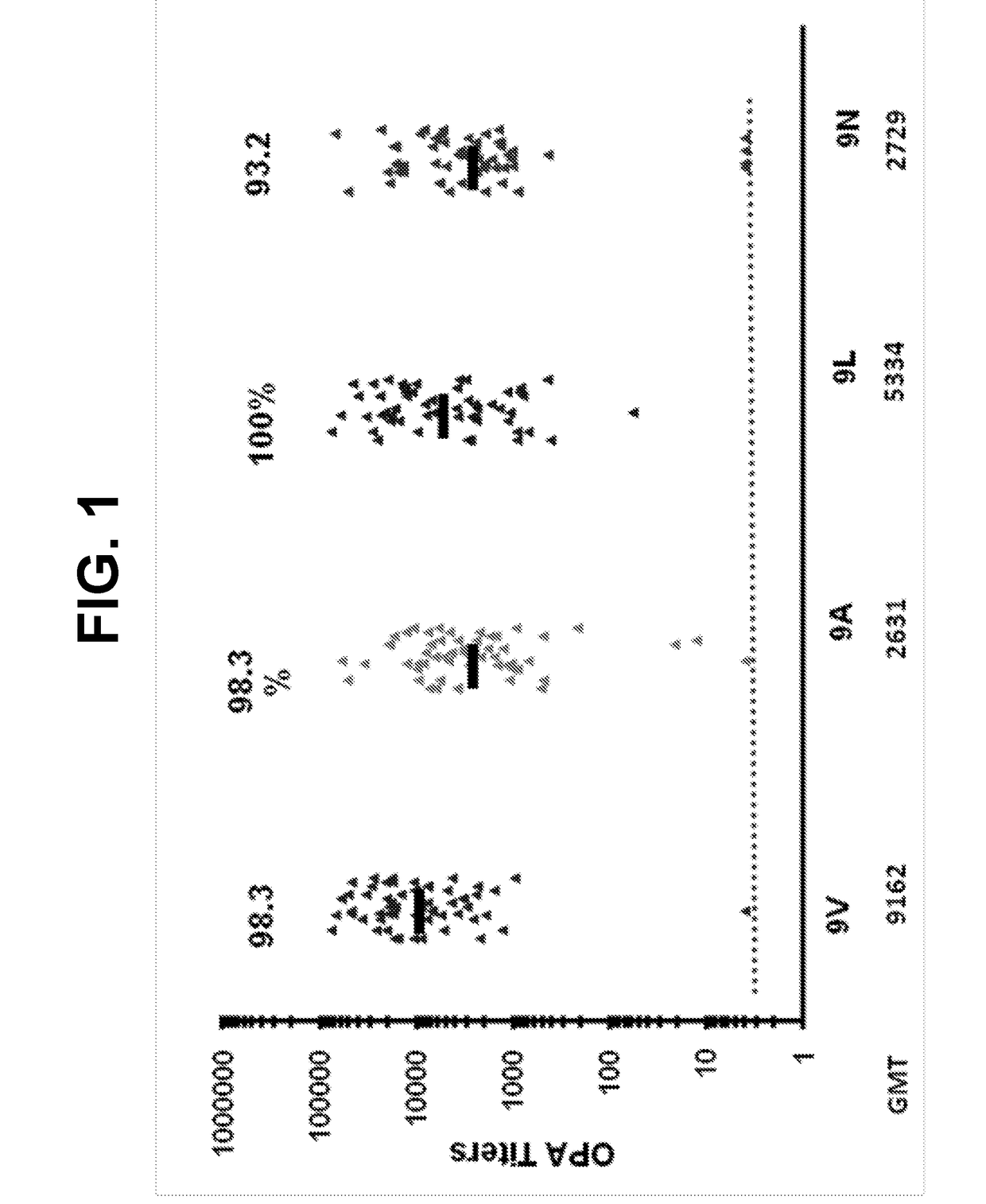
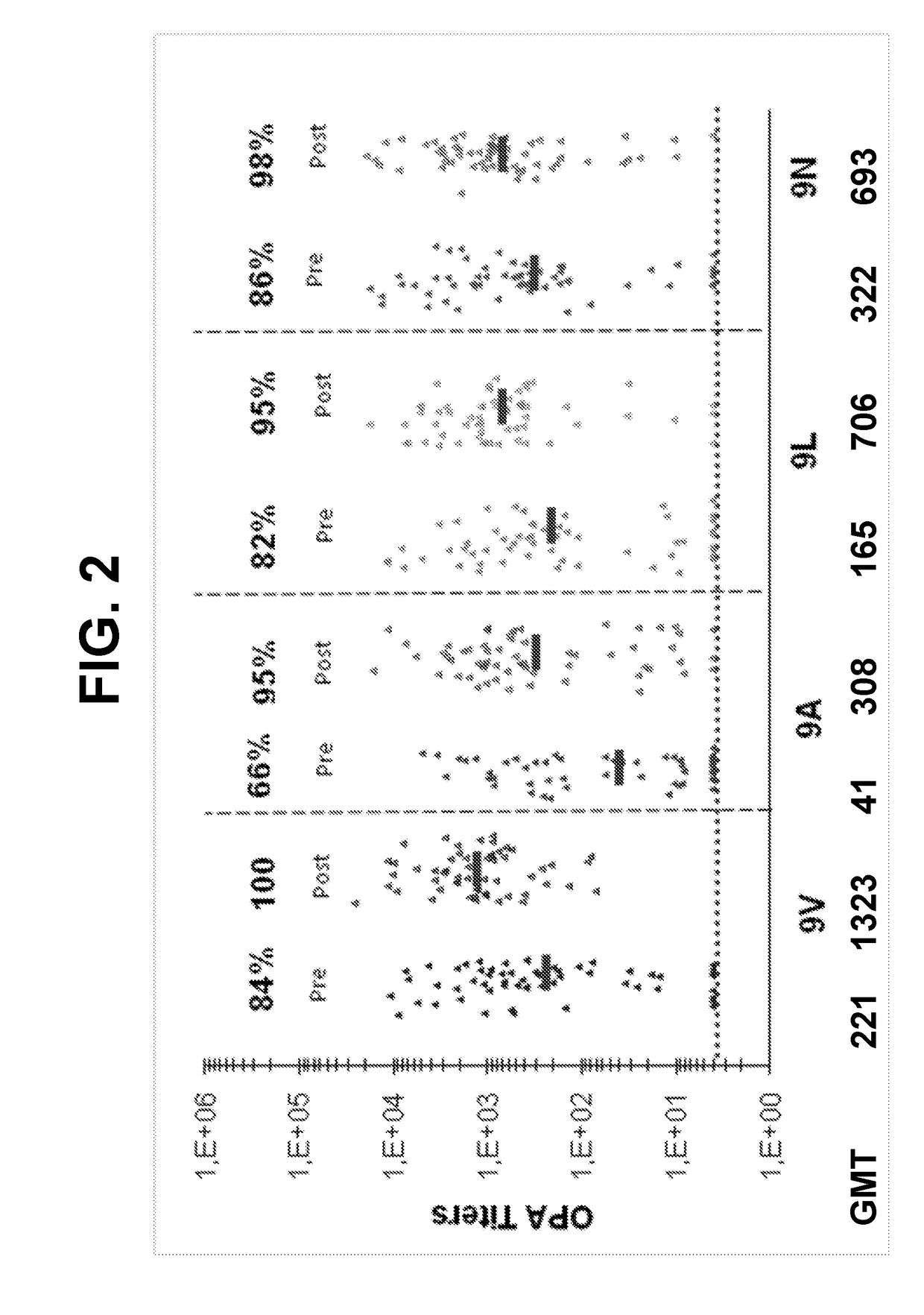
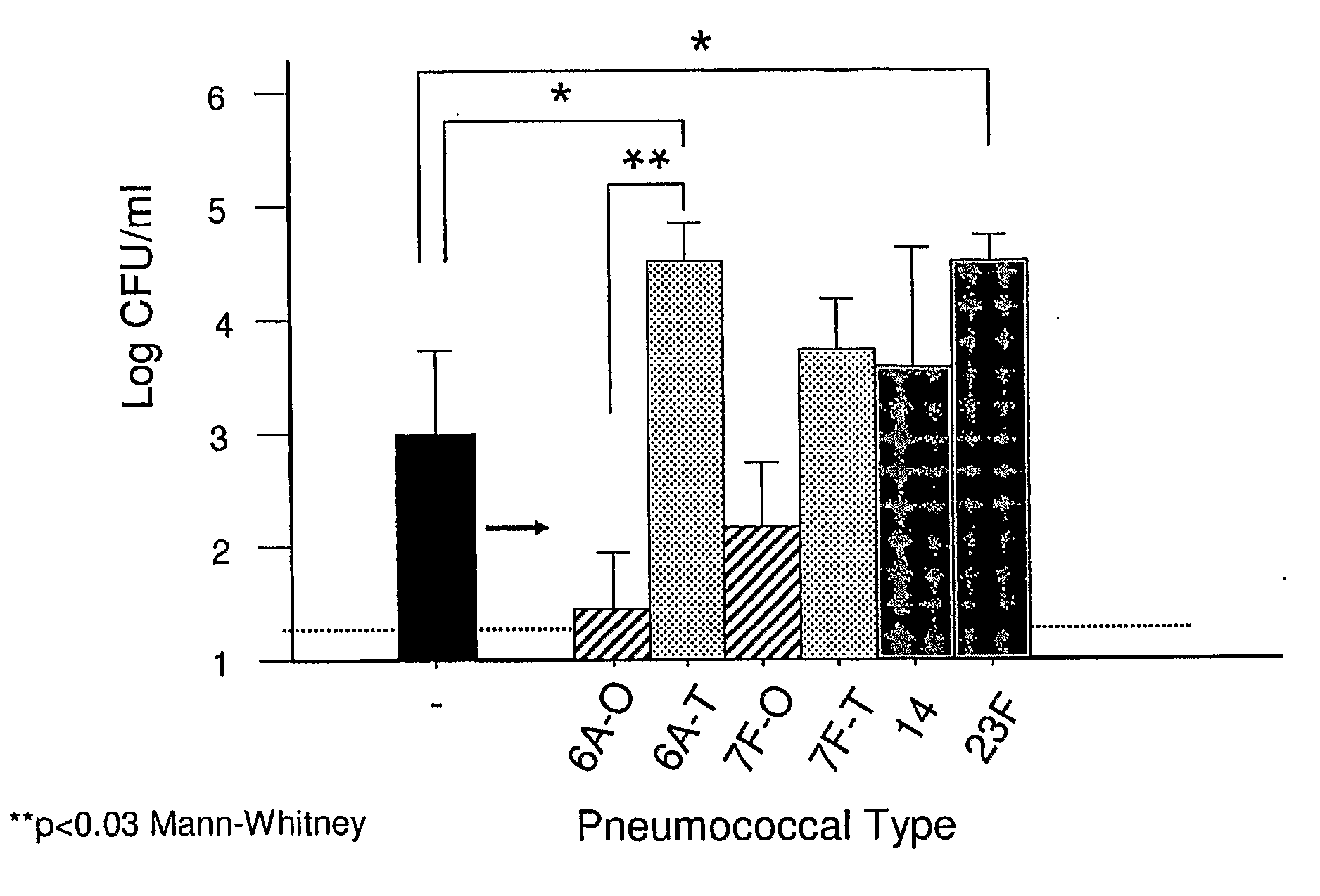
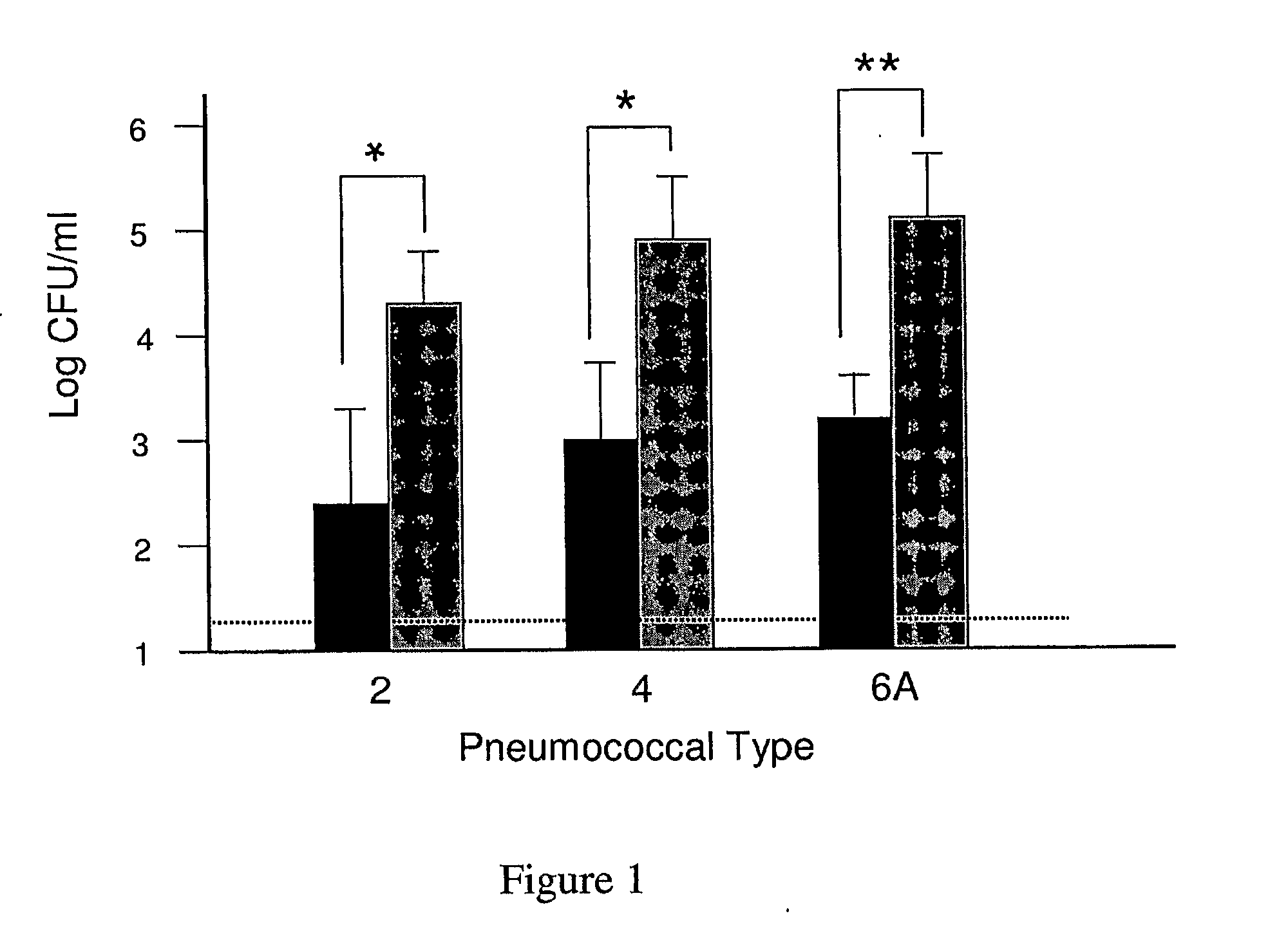
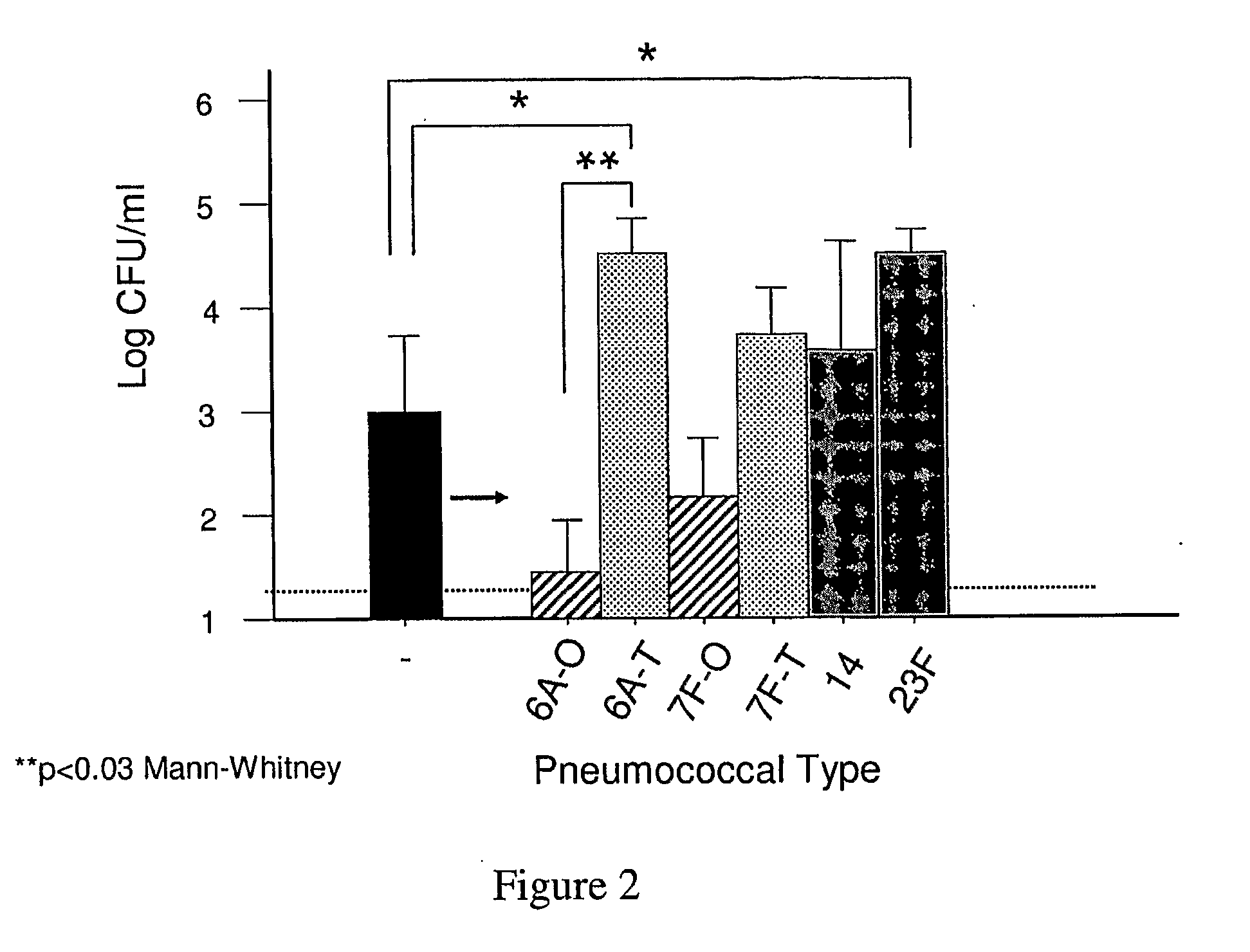
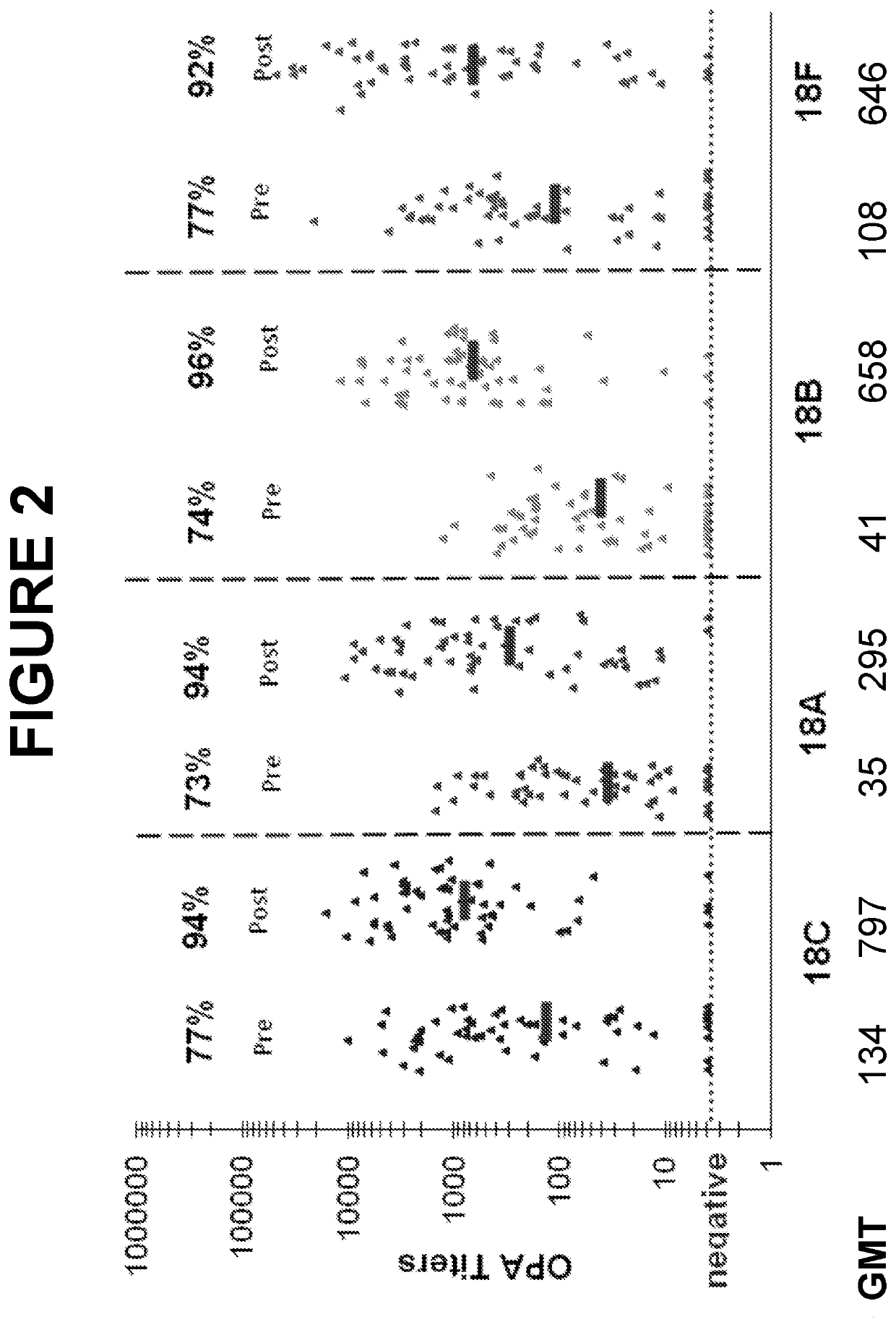
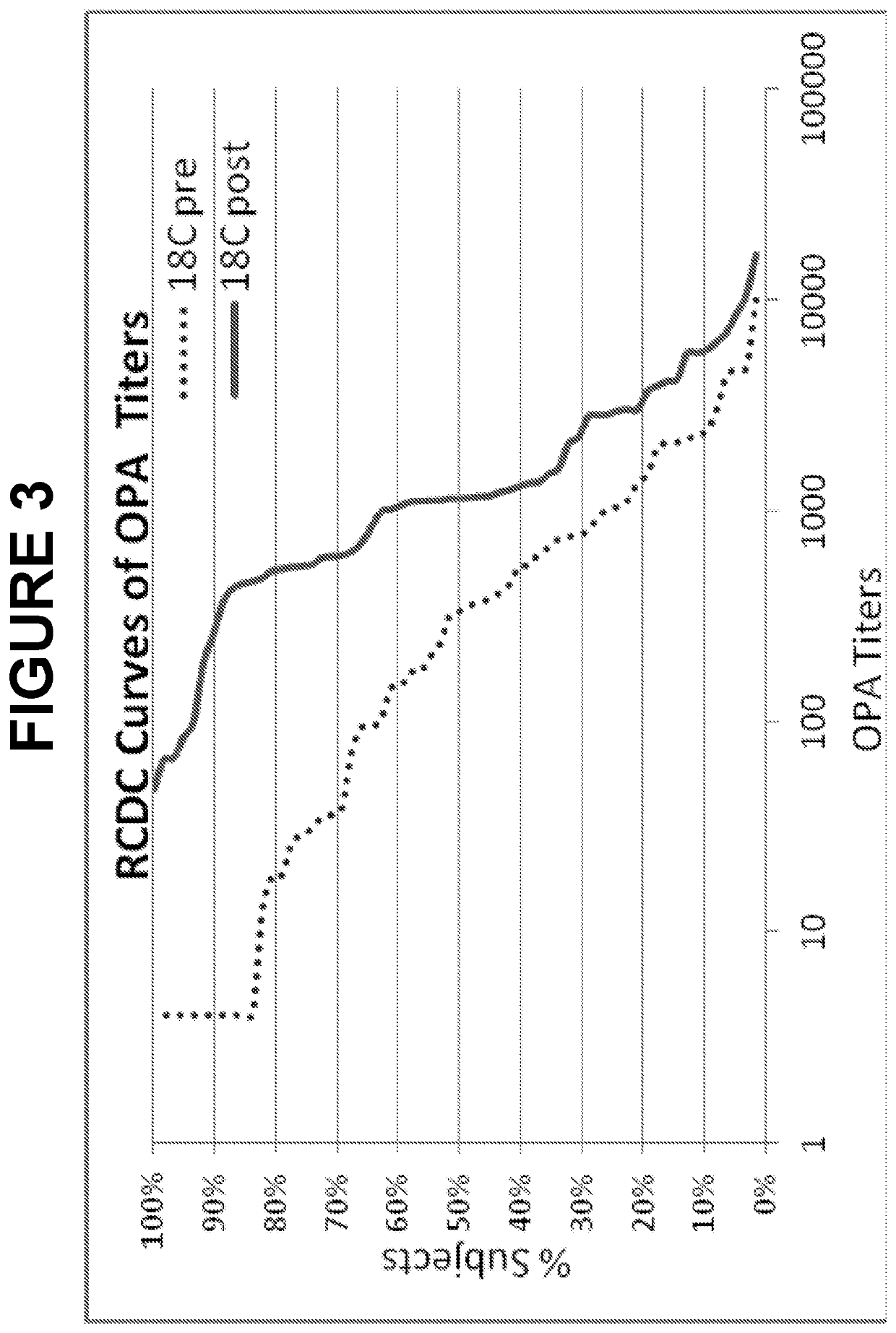
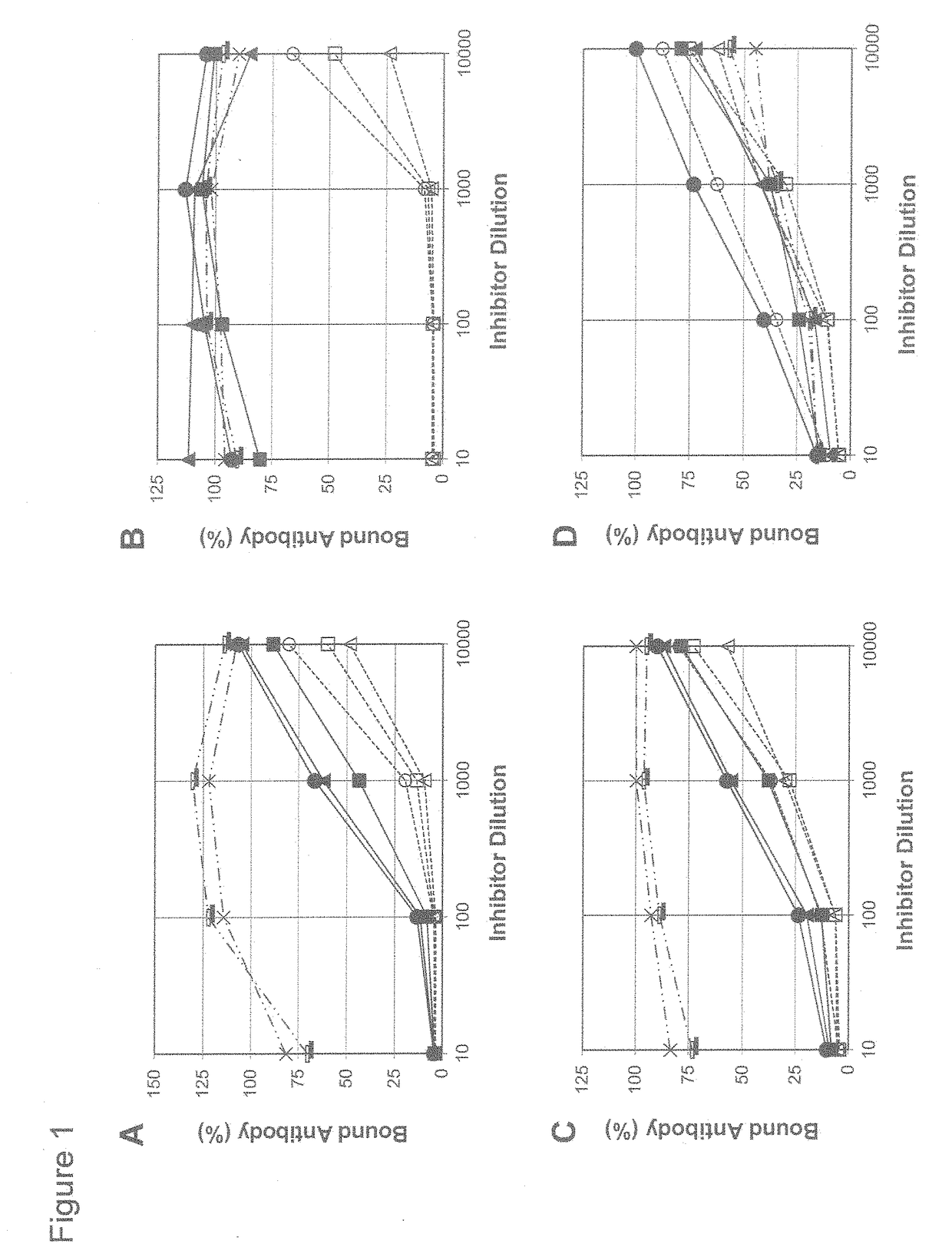
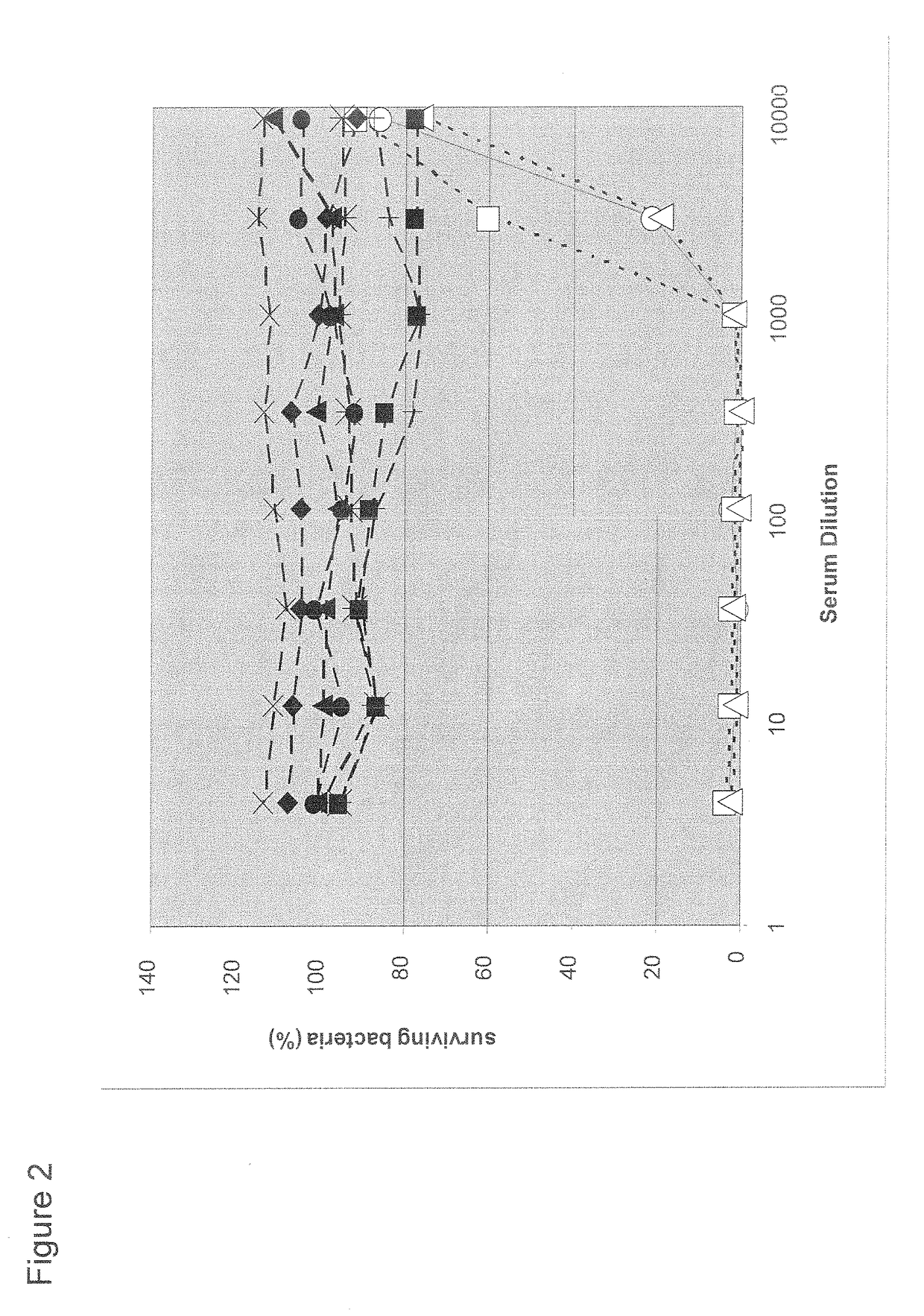
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 9, while limiting the number of conjugates. The present invention thereforerelates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Compositions and methods for administering pneumococcal DNA
Plasmid DNA encoding at least one pneumococcal antigen or epitope of interest and methods for making and using such a plasmid are disclosed and claimed. The epitope of interest can be PspA or a fragment thereof. Compositions containing the plasmid DNA are useful for administration to a host susceptible to pneumococcal infection for an in vivo response, such as a protective response, or for generating useful antibodies. The inventive plasmid can also be transfected into cells for generating antigens or epitopes of interest in vitro. And the inventive plasmid can be prepared by isolating DNA (coding for: promoter, leader sequence, epitope of interest and terminator), and performing a three-way ligation. More particularly, administration of DNA encoding pneumococcal antigens or epitopes of interest and compositions therefor for eliciting and immunological response against S. pneumoniae, such as a protective response preventive of pneumococcal infection, are disclosed and claimed. Thus, pneumococcal vaccines or immunological compositions, and methods of making and using them, are disclosed and claimed.
Owner:BRILES DAVID E +2
Pneumococcal serotypes
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {→2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:INST ADOLFO LUTZ +1
Pneumococcal serotypes
InactiveUS20130315958A1Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Pneumococcal vaccine and uses thereof
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumoccocal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARMA GRP INC
Live, attenuated pneumococcal vaccine
InactiveUS20100021498A1Accurate lossEffectively colonizing the subject's nasopharynxBacterial antigen ingredientsBacteriaCoccidiaPneumococcal vaccine
This invention relates to a live mutated strain of S. pneumoniae which is incapable of expressing polysaccharide capsule, while still capable of colonizing the nasopharynx of the subject.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Immunogenic Compositions for Use in Pneumococcal Vaccines
ActiveUS20190343946A1Antibacterial agentsBacterial antigen ingredientsStreptococcus mitisImmunogenicity
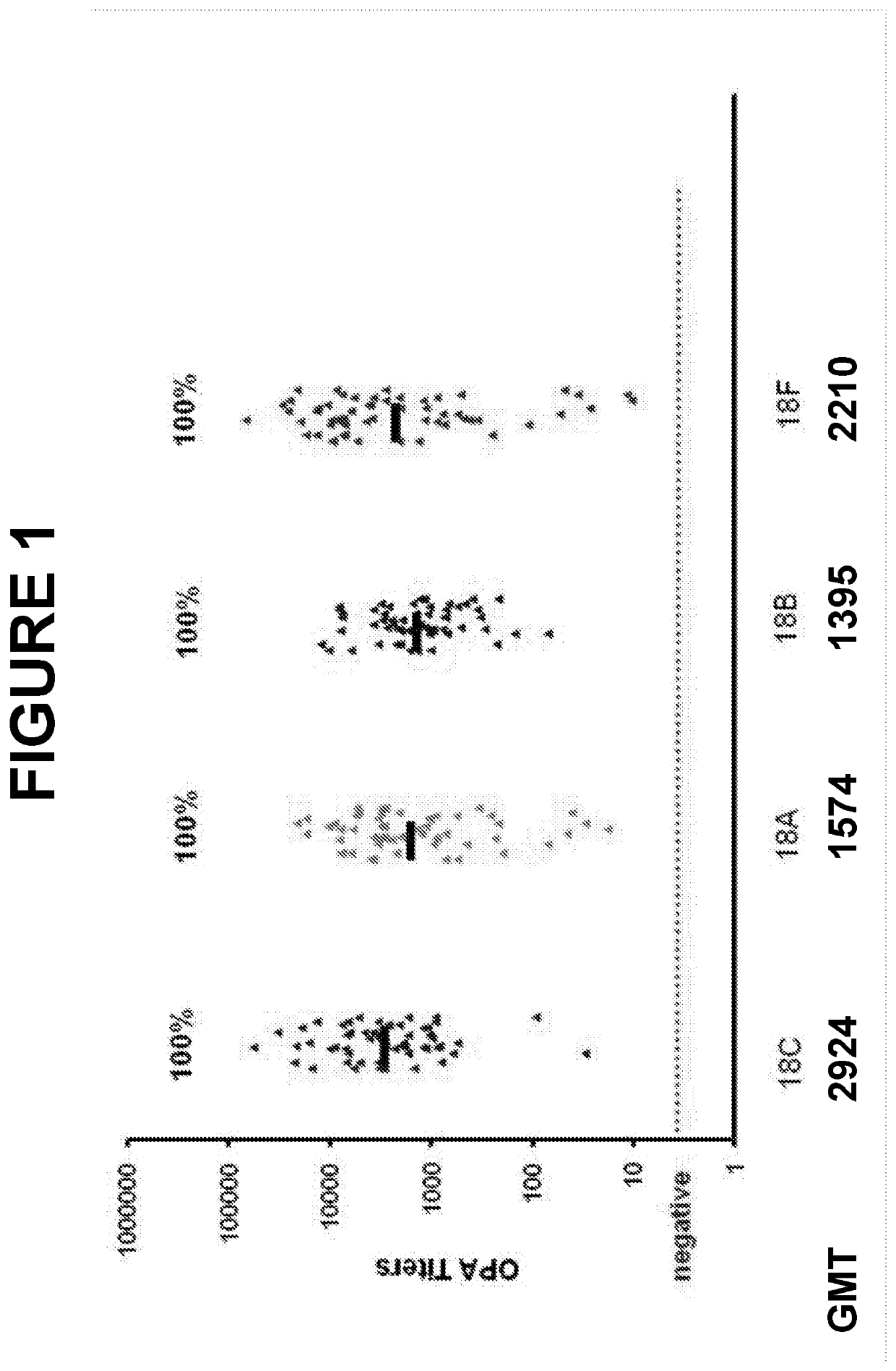
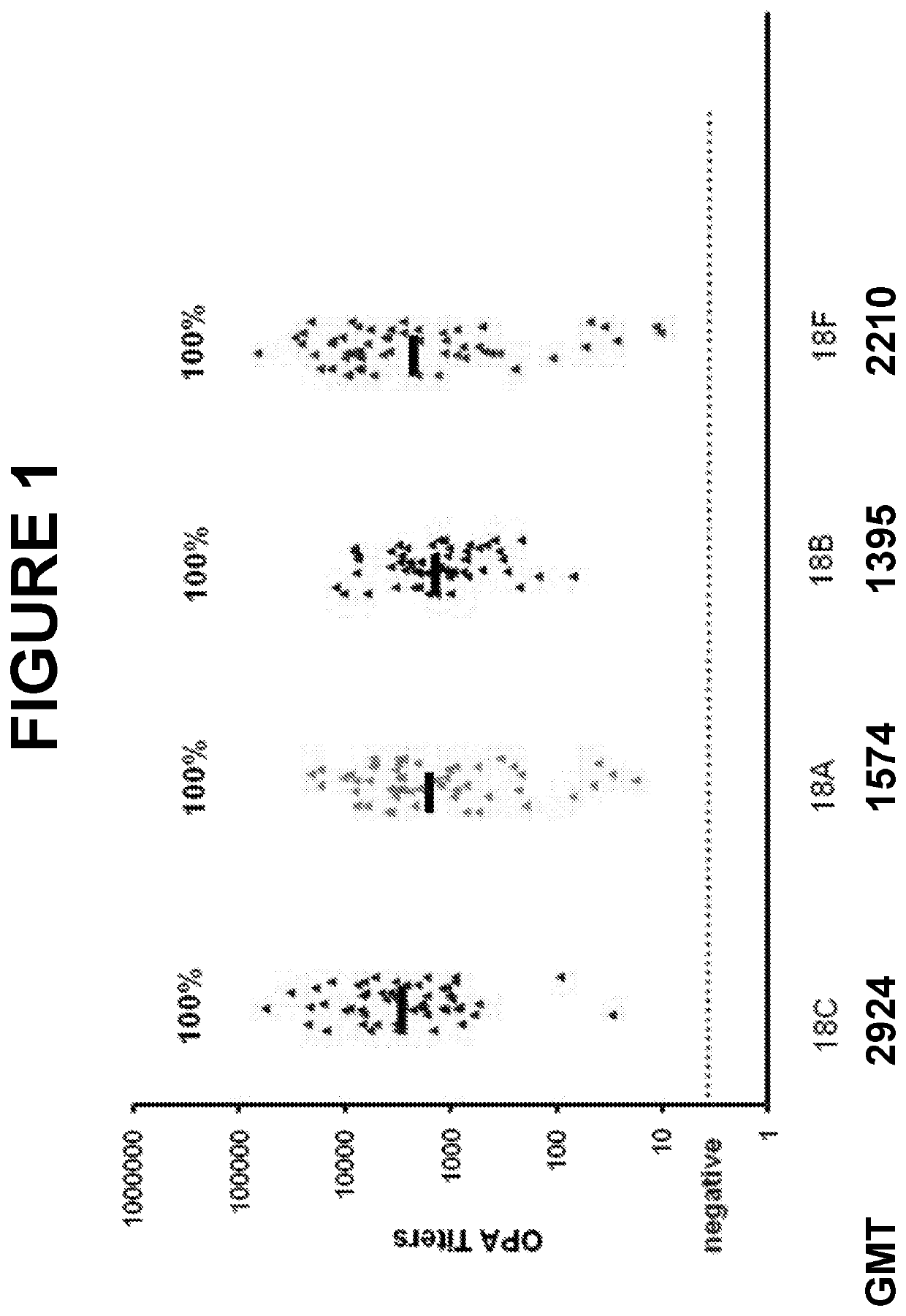
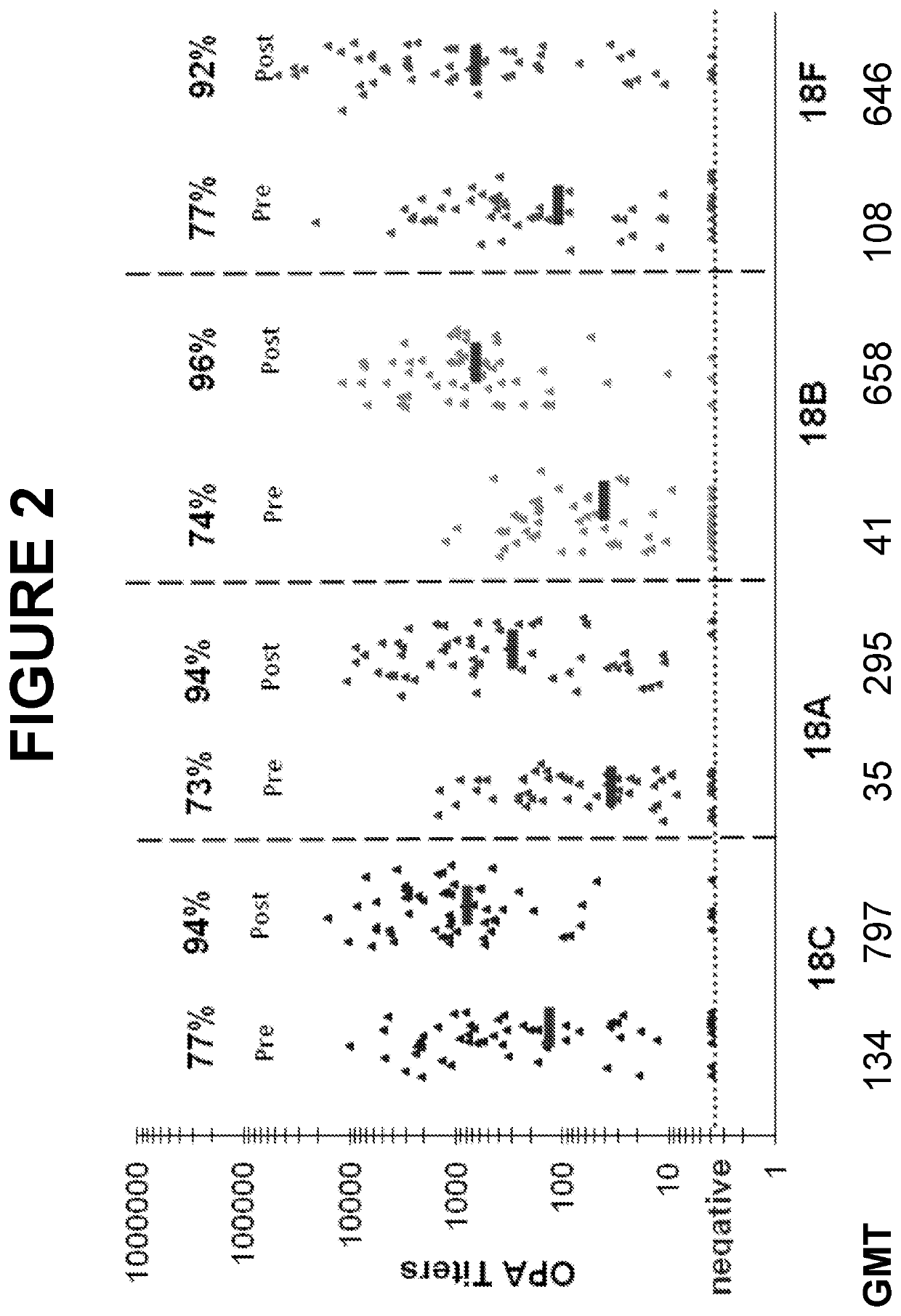
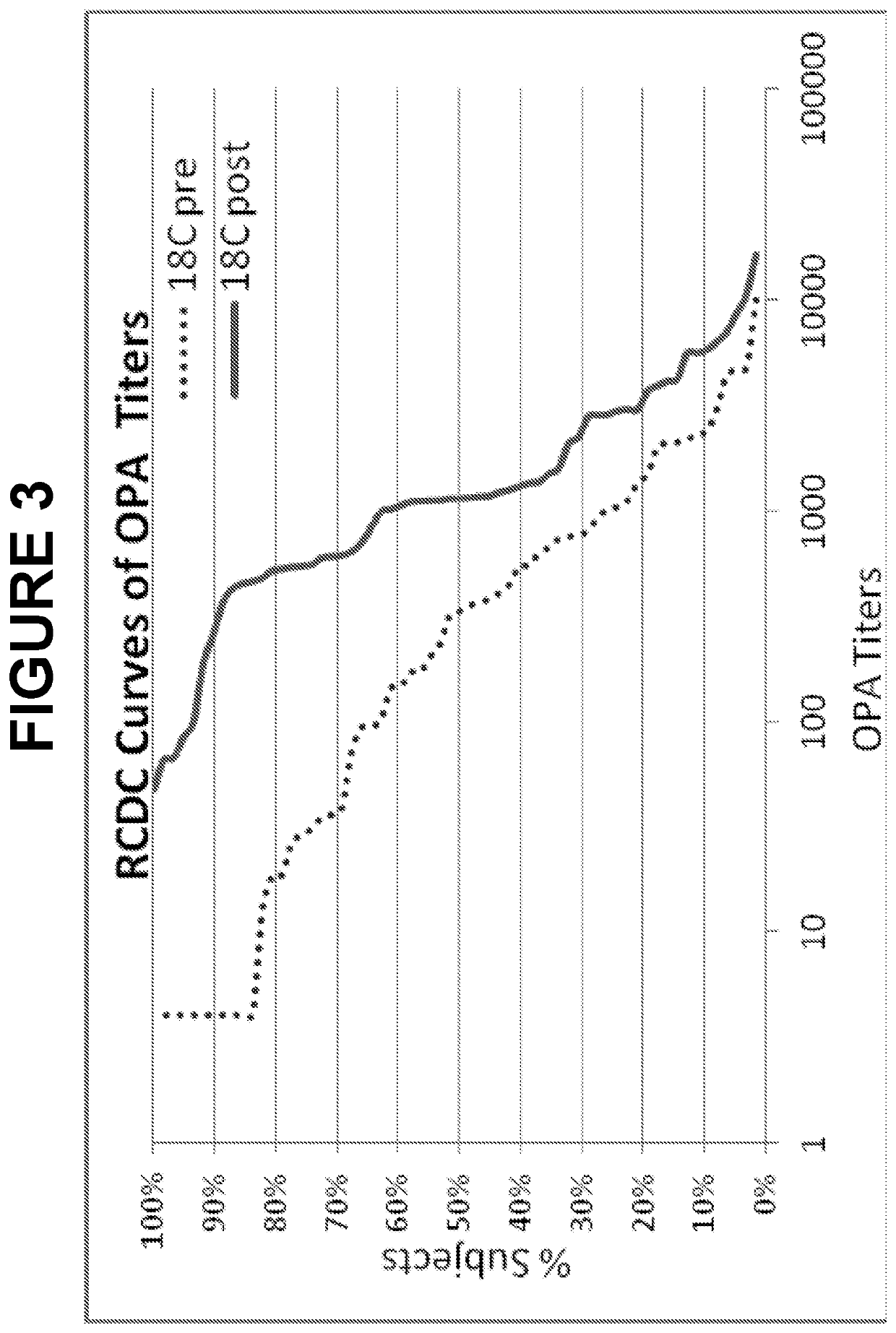
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 18, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Pneumococcal serotypes
InactiveUS9778266B2Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Pneumococcal vaccine containing pneumococcal surface protein a
ActiveUS20150320851A1Induce immune responseInduce protective immunityAntibacterial agentsBacterial antigen ingredientsCoccidiaProtein s antigen
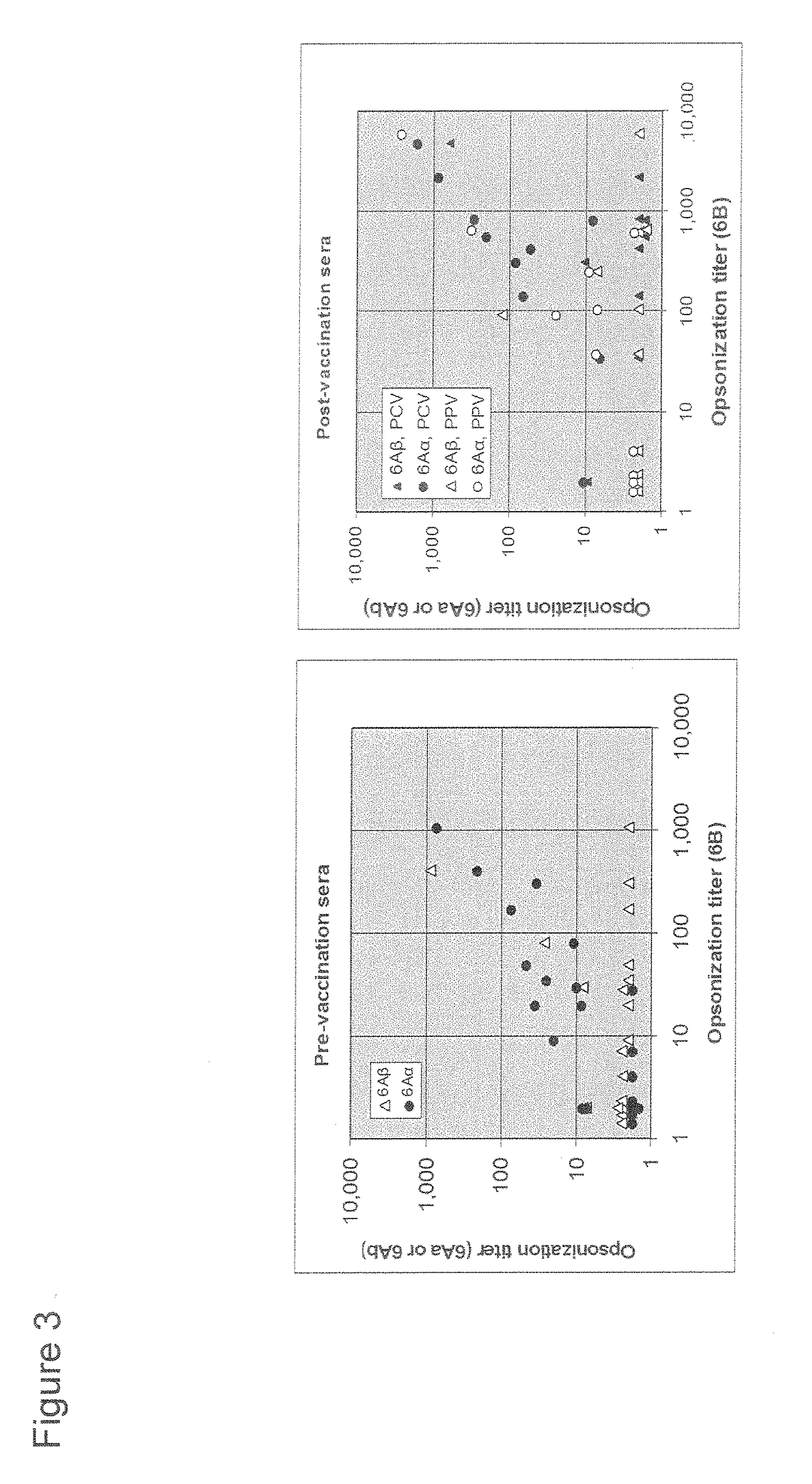
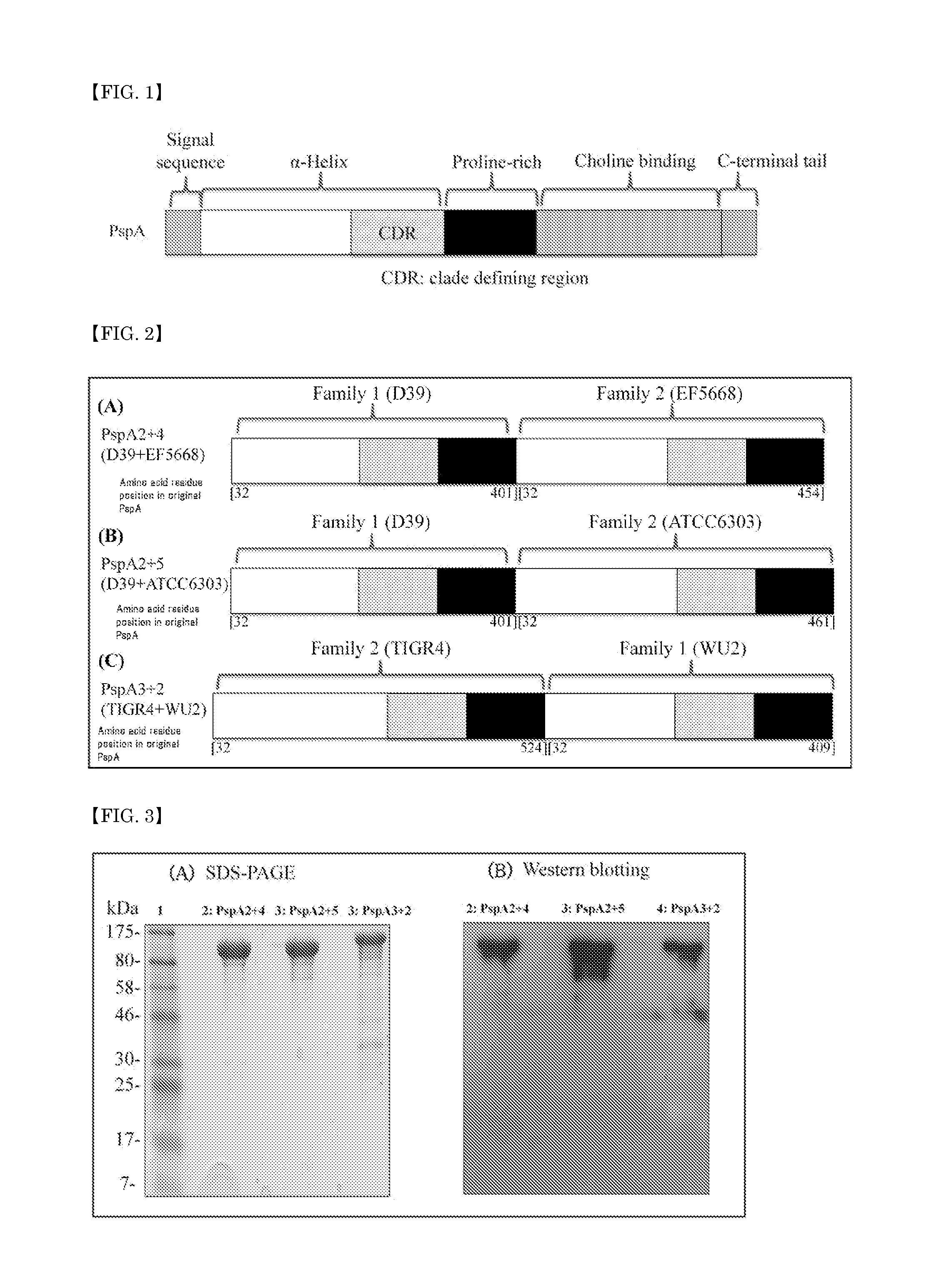
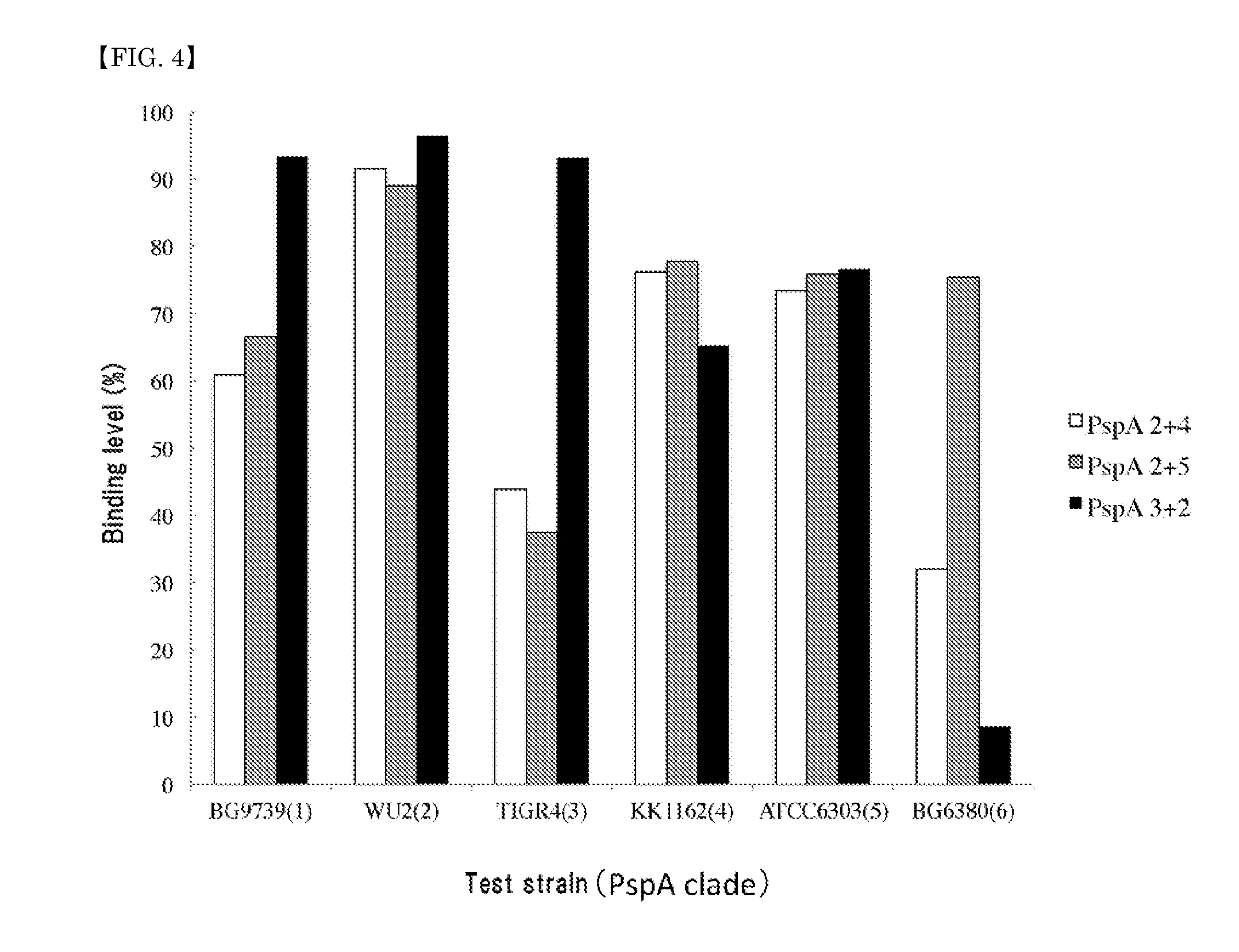
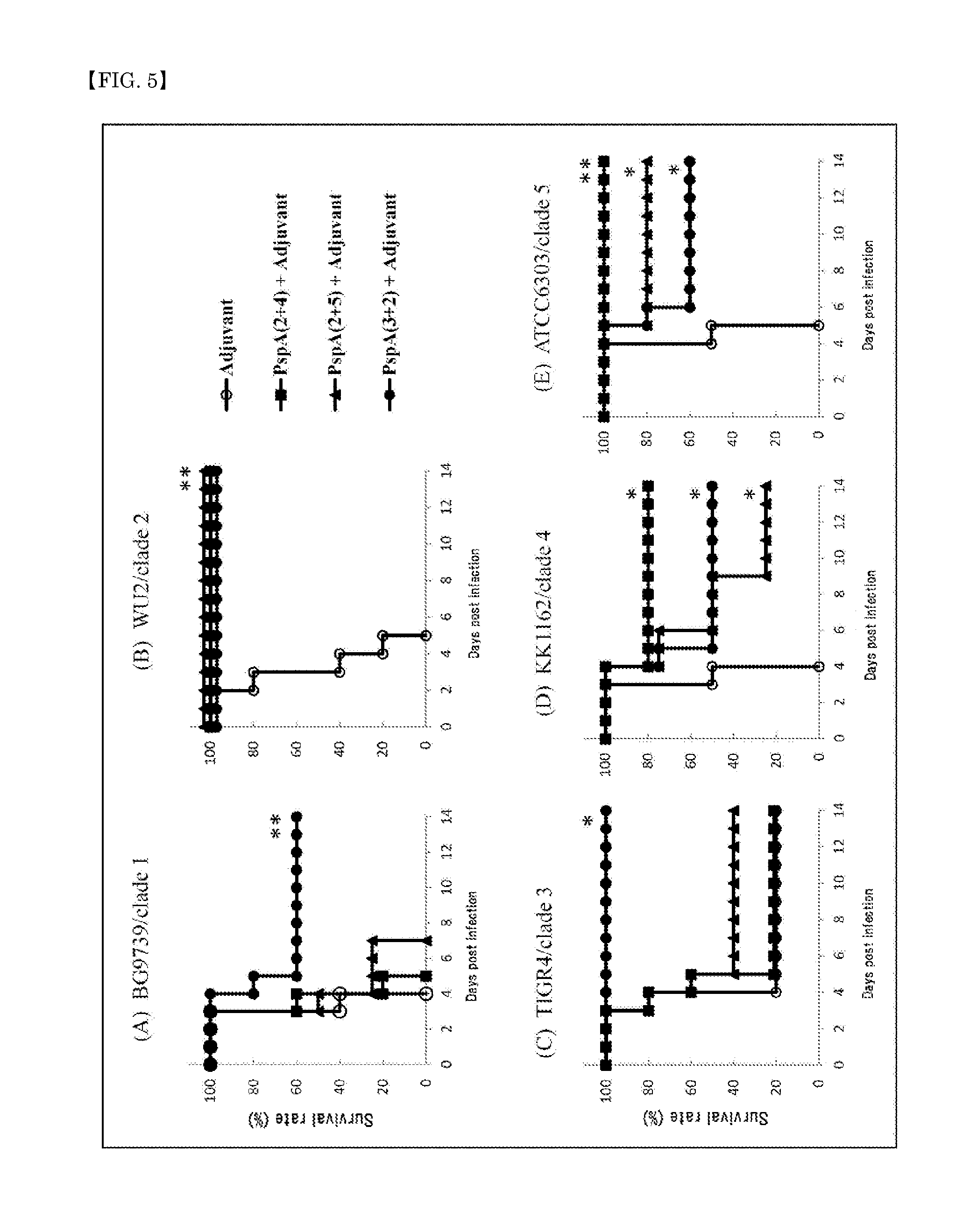
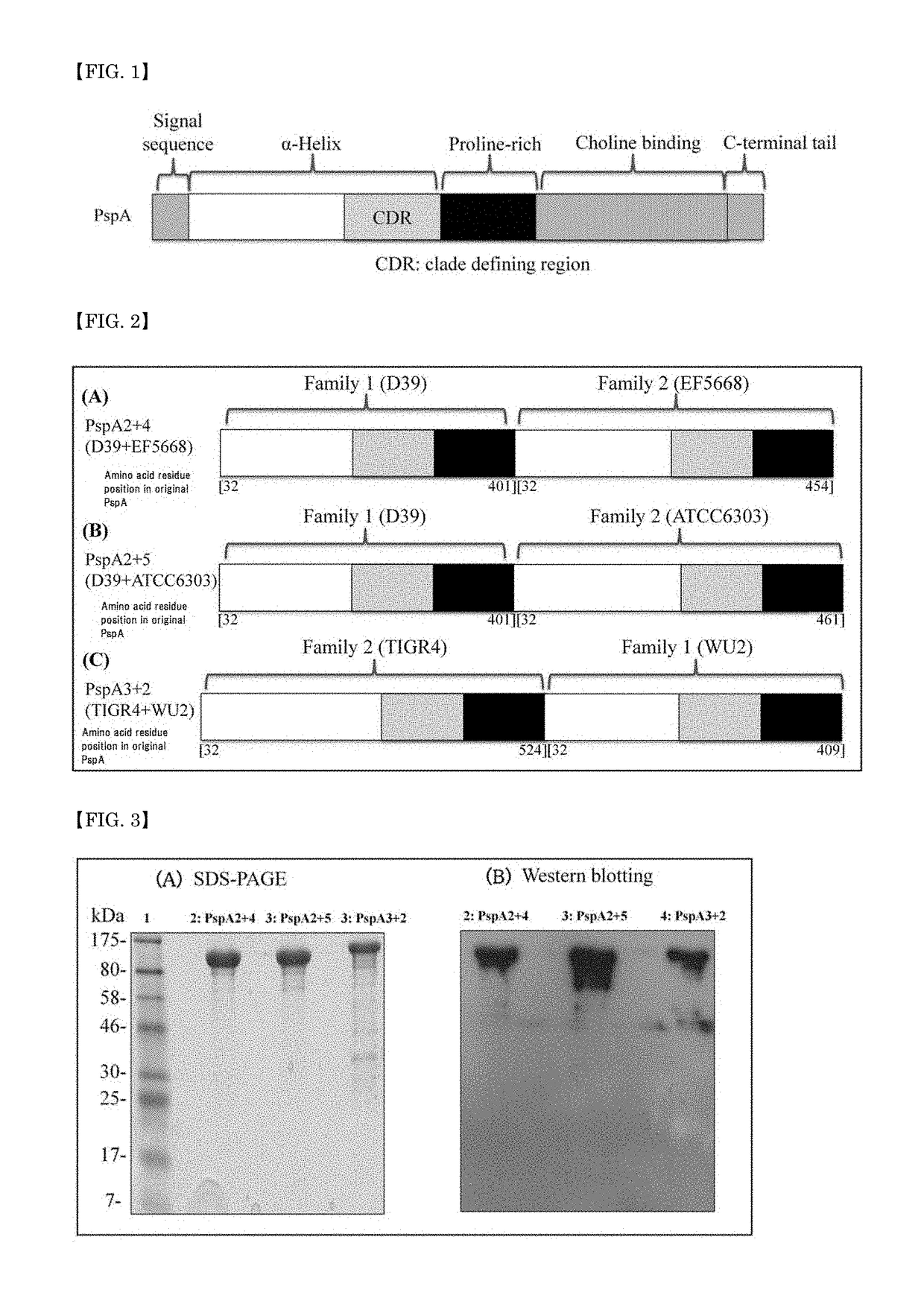
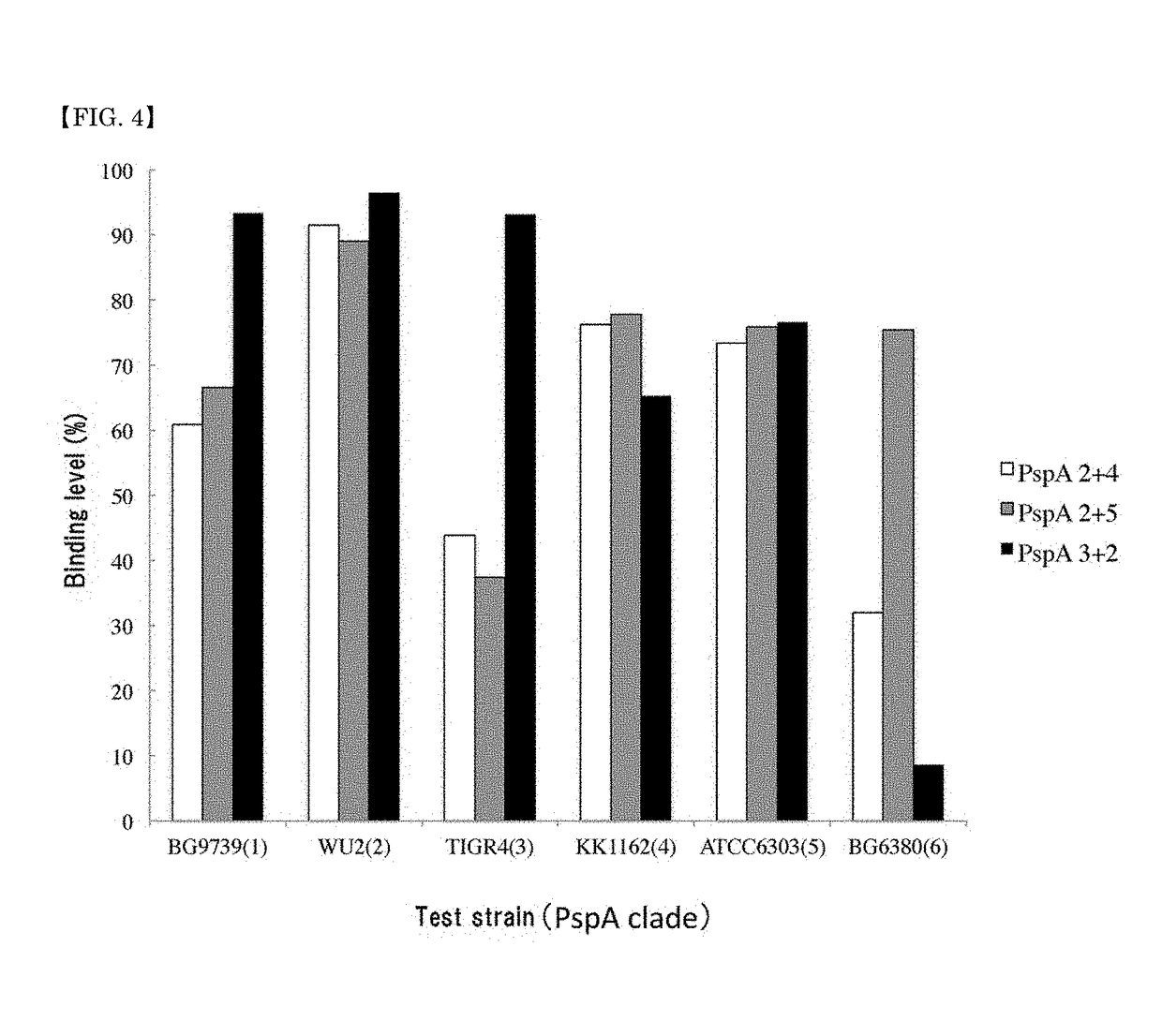
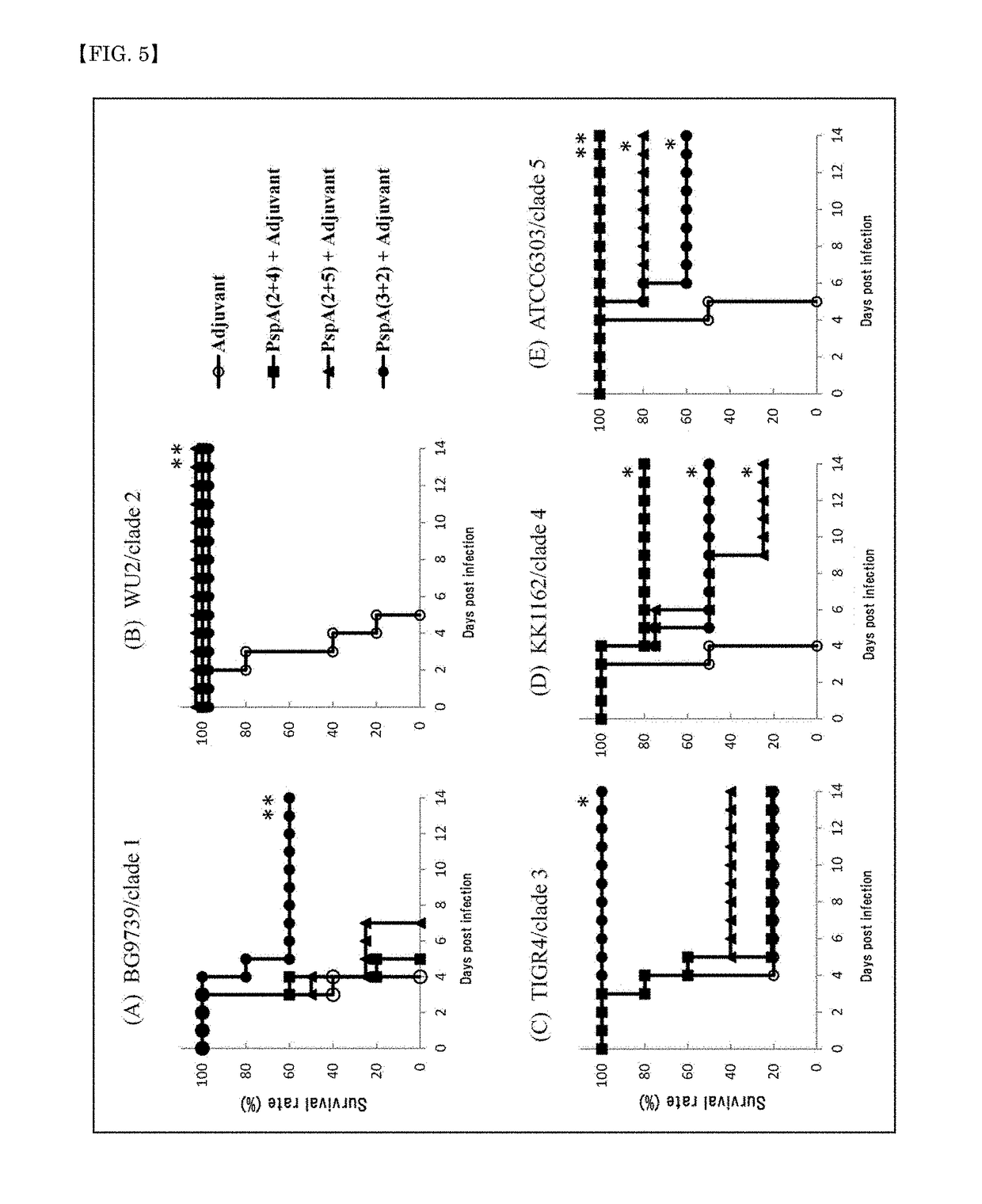
A pneumococcal vaccine comprising a fusion protein at least comprising a full-length family 1 pneumococcal surface protein A (PspA) or a fragment thereof, and a full-length family 2 PspA or a fragment thereof, in particular any one of the following fusion proteins (1) to (3):(1) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 3 PspA,(2) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 4 PspA, and(3) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 5 PspA,is useful as a pneumococcal vaccine comprising a single protein antigen that has broadly cross-reactive immunogenicity and can induce immune response against a wide range of pneumococcal clinical isolates.
Owner:OSAKA UNIV
Pneumococcal serotype 6D
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6D, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit →2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→4) ribitol (5→phosphate. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Pneumococcal serotypes
ActiveUS20180136224A1Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Owner:UAB RES FOUND +1
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS20030175293A1Improve efficiencySufficient quantityAntibacterial agentsBiocideBacteroidesPassive Immunizations
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
Pneumococcal serotypes
ActiveUS20100143414A1Antibacterial agentsBacterial antigen ingredientsPneumococcal serotypesAntiendomysial antibodies
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {→2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:INST ADOLFO LUTZ +1
Immunogenic compositions for use in pneumococcal vaccines
ActiveUS20190000952A1Raise the ratioIncrease OPA titerBacterial antigen ingredientsAntiinfectivesCoccidiaSalmonella serotype typhi
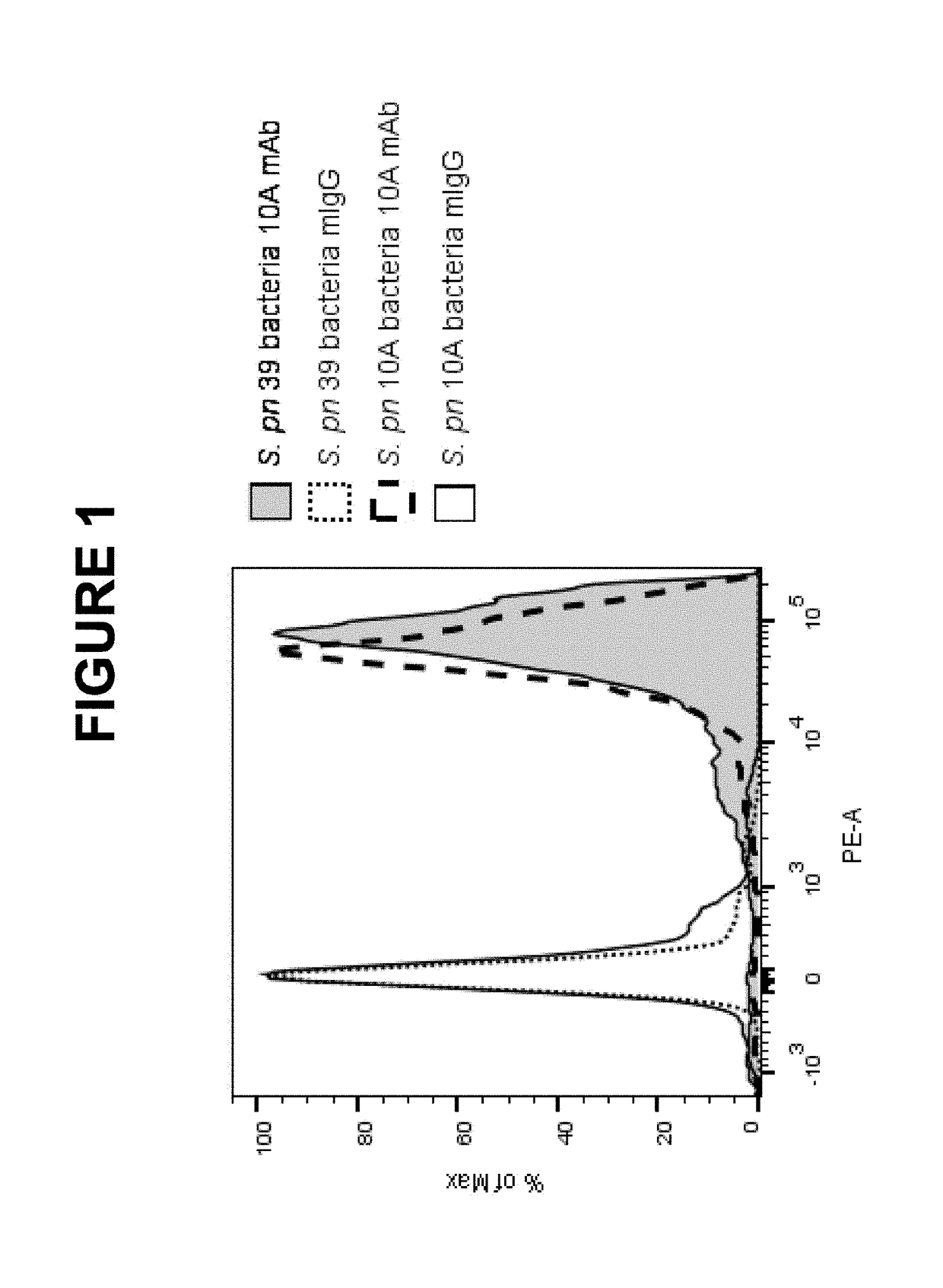
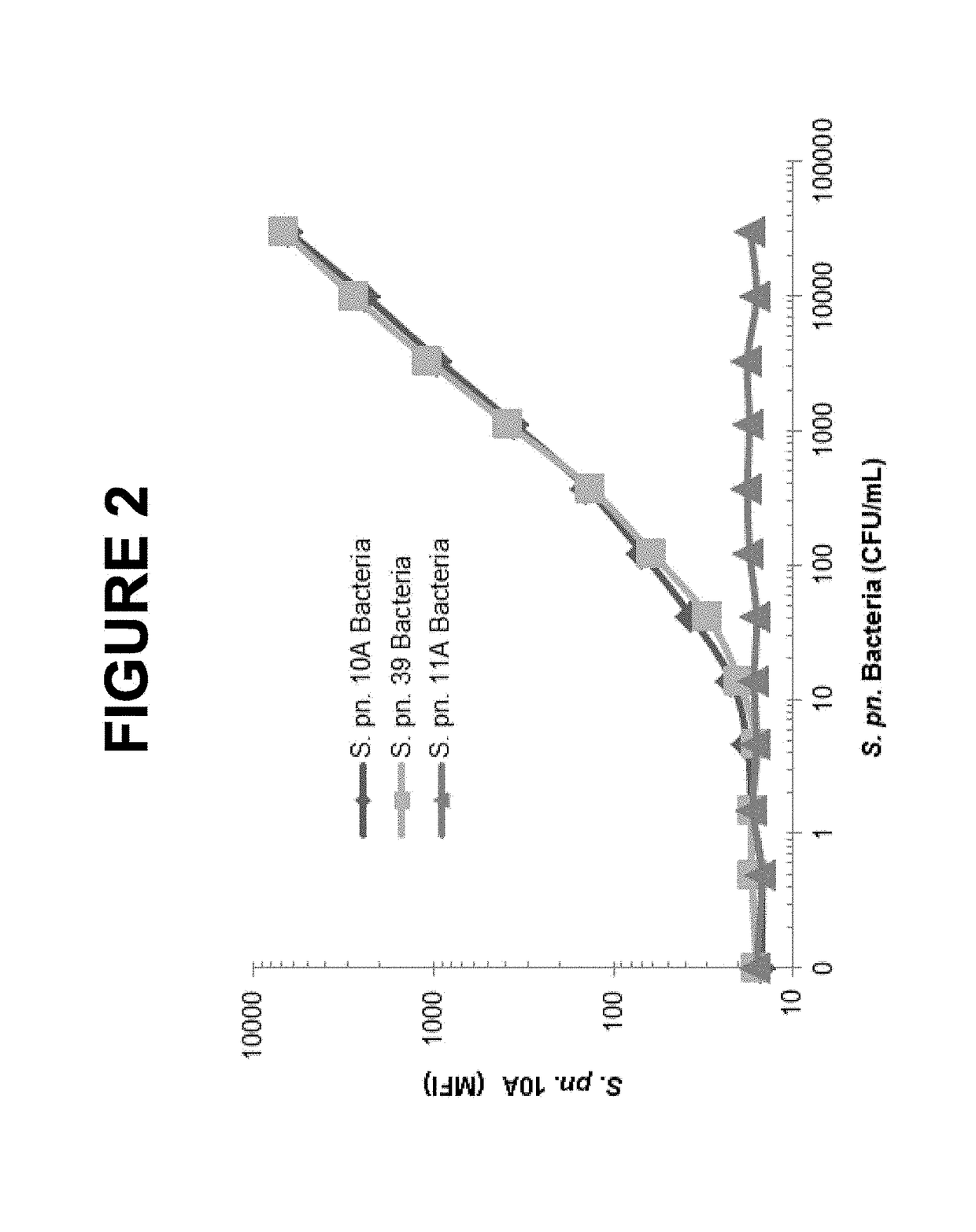
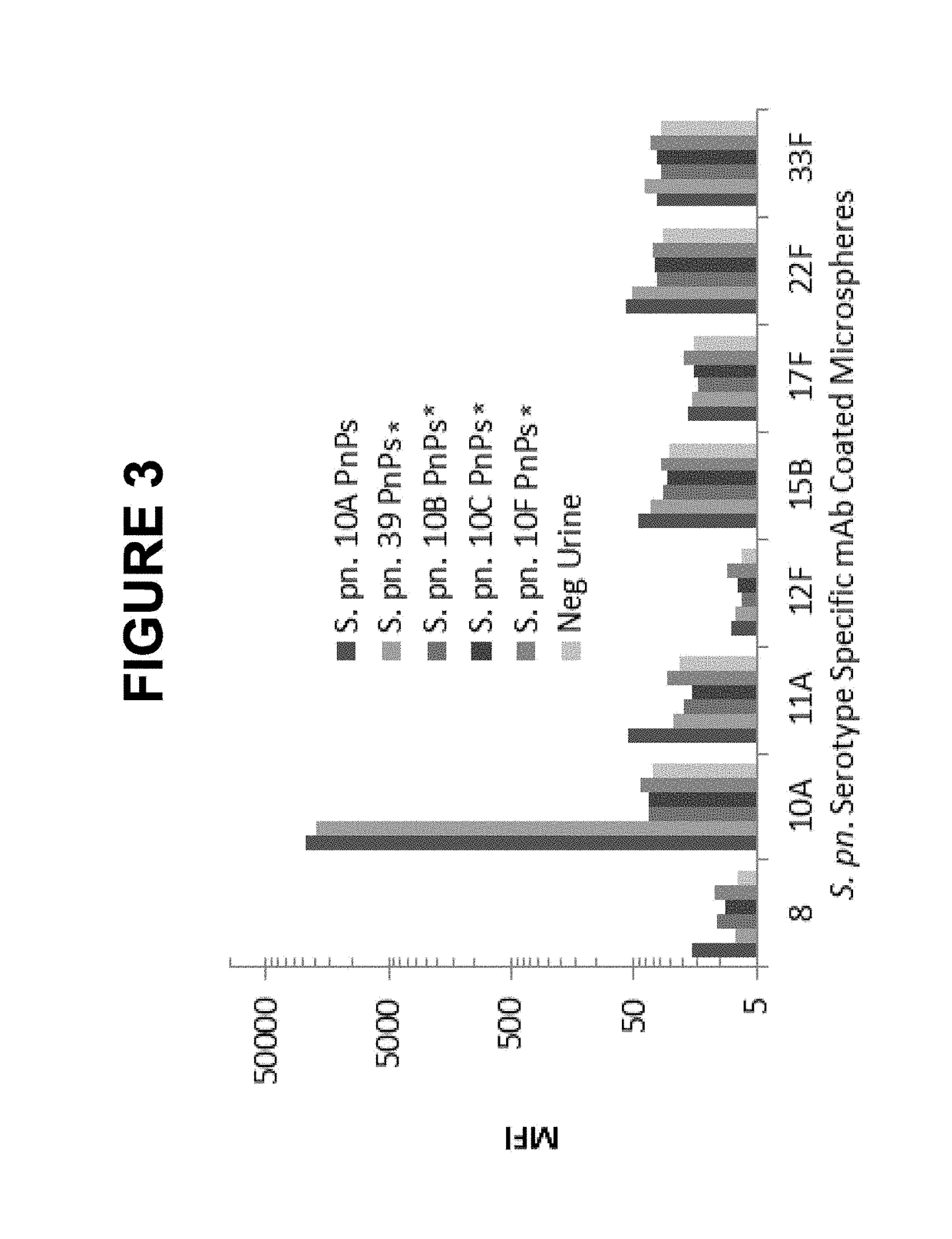
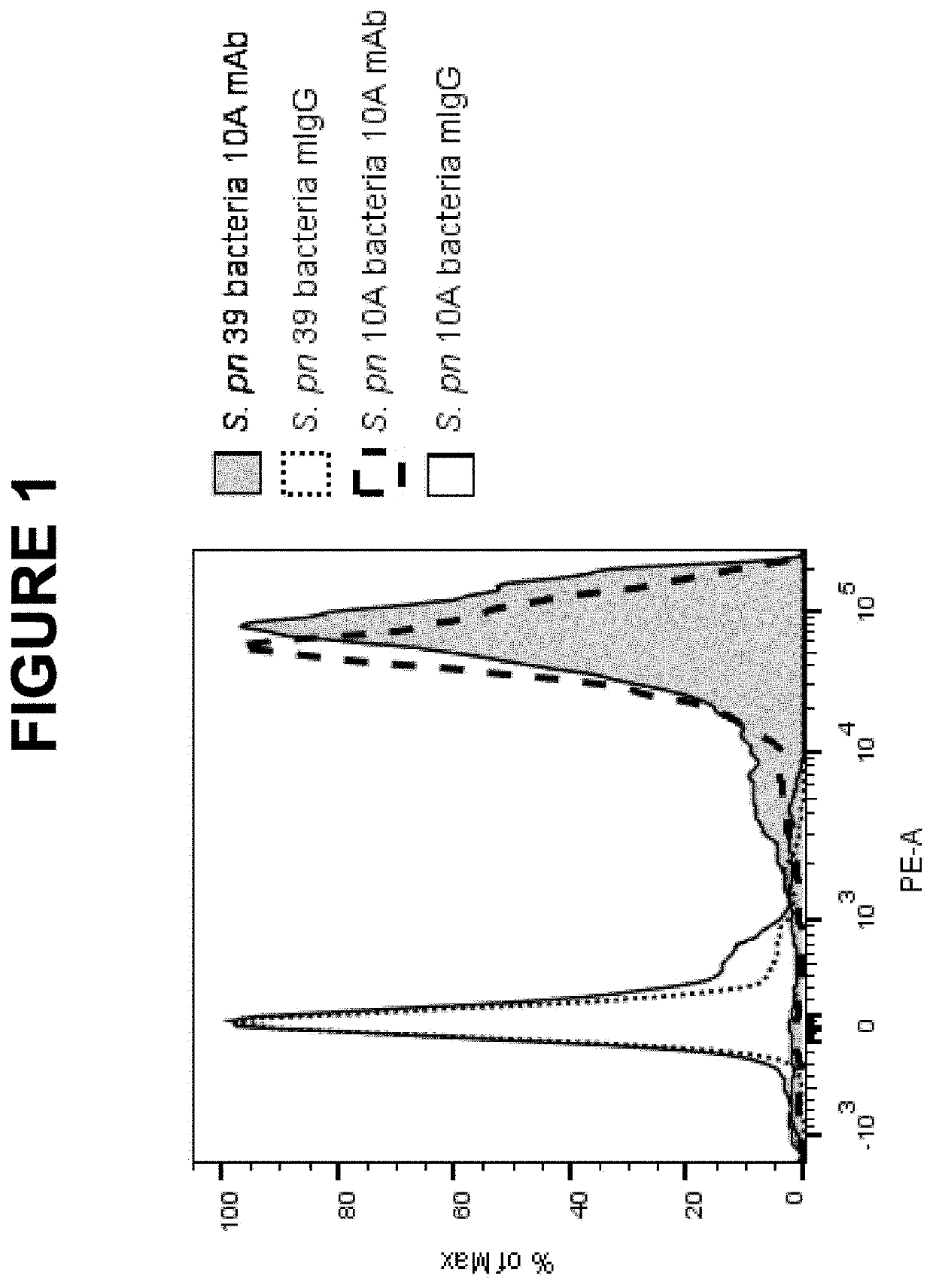
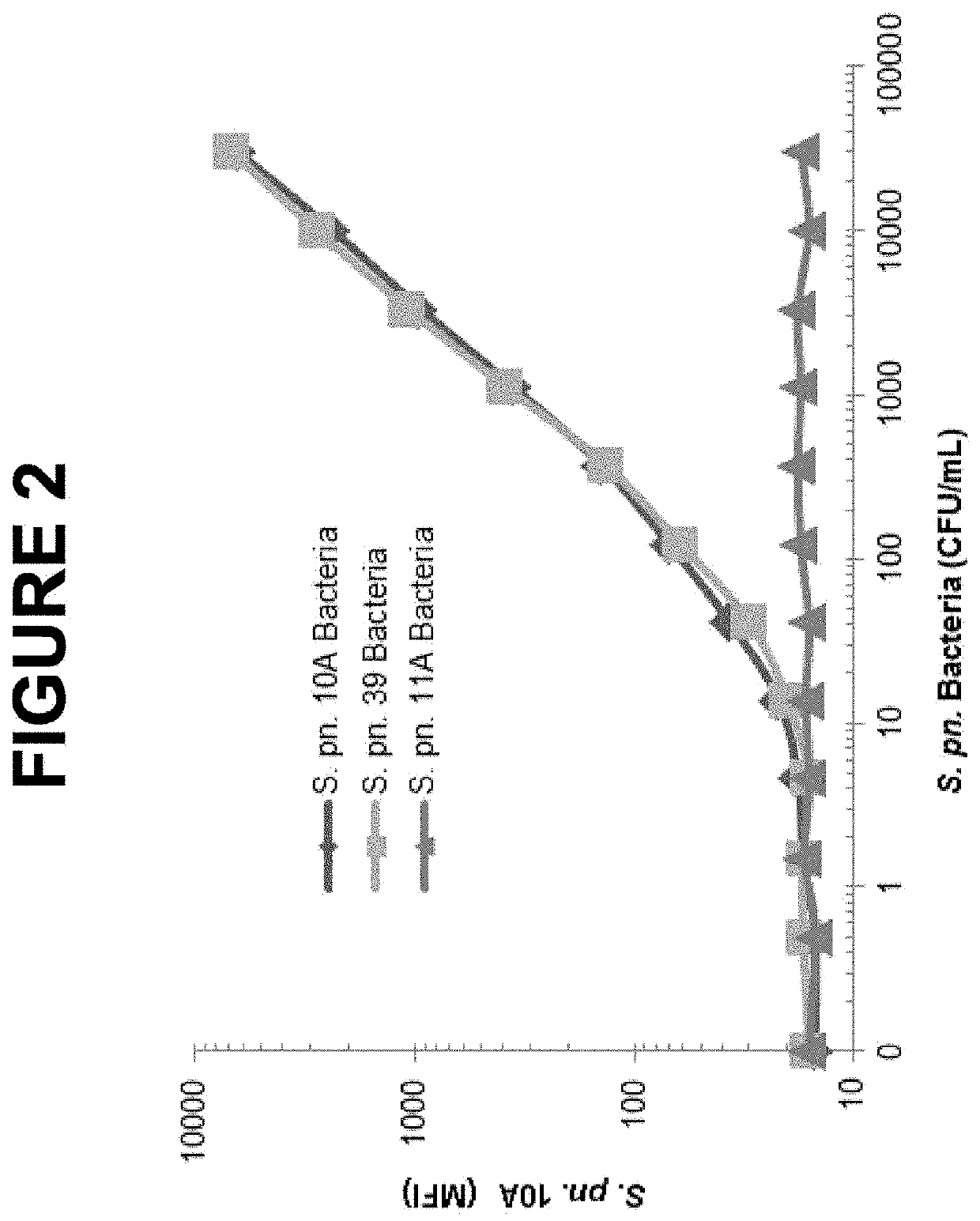
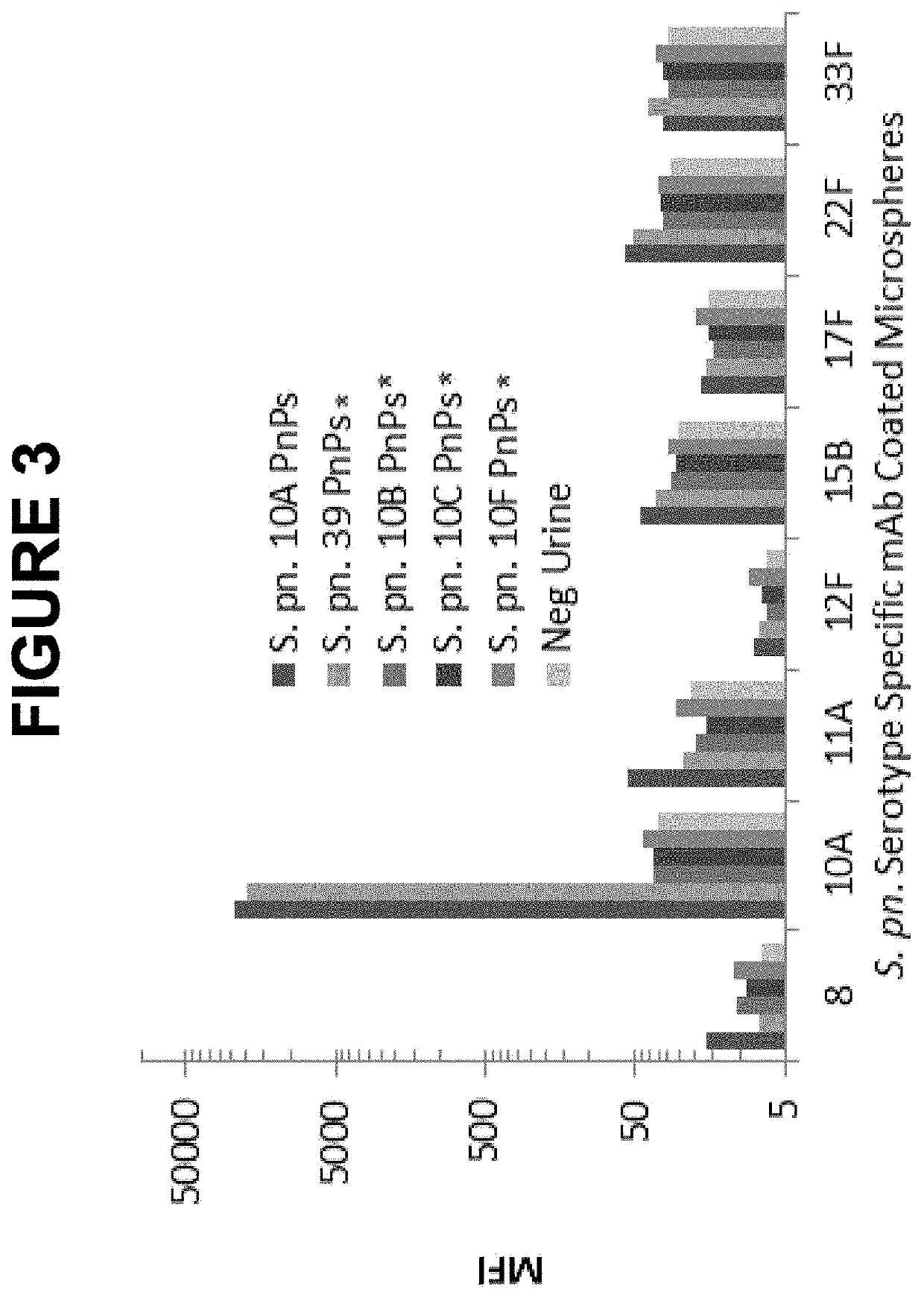
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Immunogenic compositions for use in pneumococcal vaccines
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Pneumococcal vaccine containing pneumococcal surface protein A
ActiveUS9938326B2Induce immune responseInduce protective immunityAntibacterial agentsBacterial antigen ingredientsProtein antigenPneumococcal surface protein A
Owner:OSAKA UNIV
Meningococcal and pneumococcal conjugate vaccine and method of using same
ActiveUS8003112B2Efficient infectionLow costAntibacterial agentsBacterial antigen ingredientsCoccidiaMeningococcal carriage
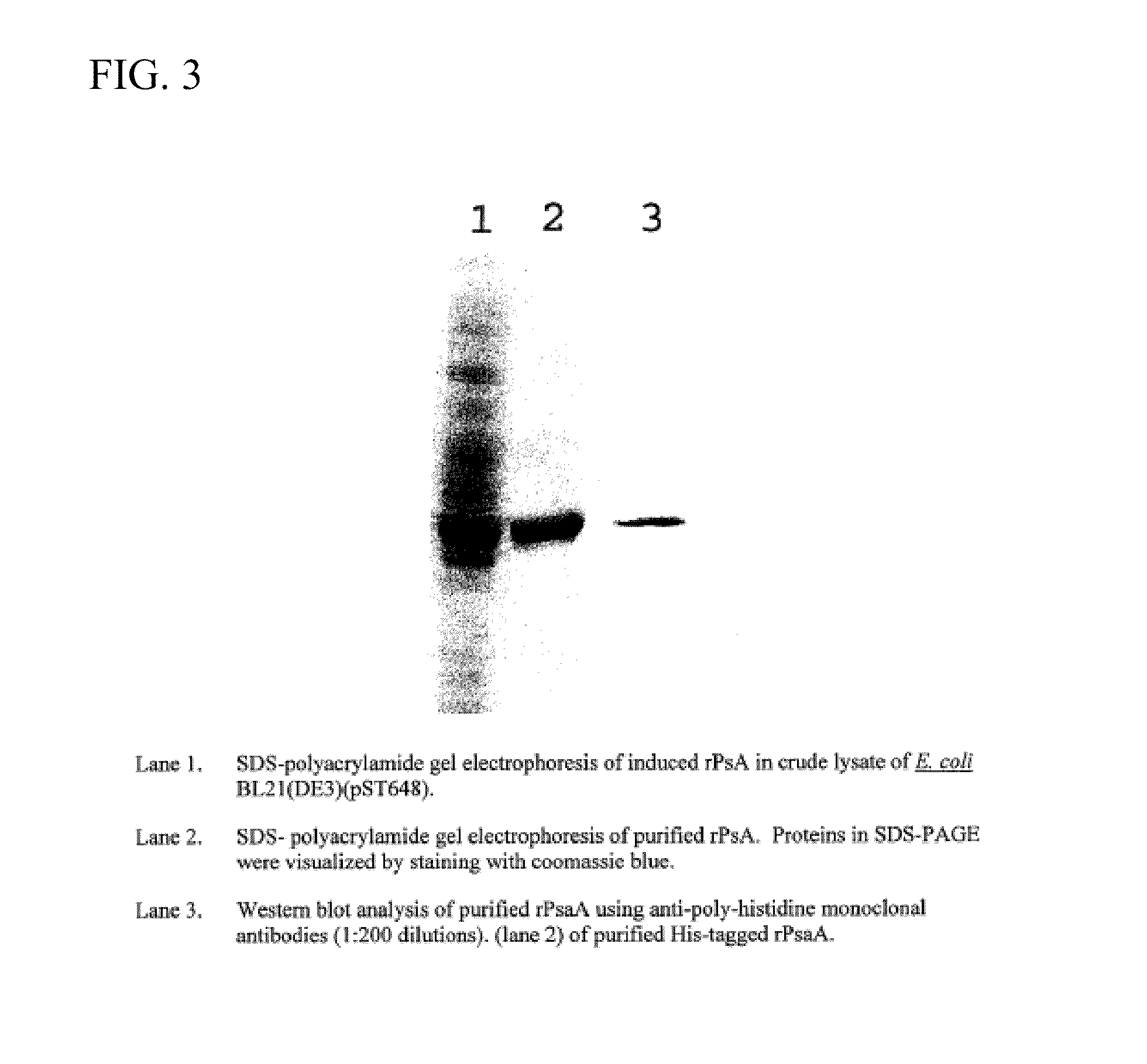
This disclosure relates to vaccine formulations comprising an immunogenic composition for inducing antibodies to both S. pneumoniae and N. meningitides in a subject. In a preferred aspect, the immunogenic composition comprises covalently conjugated recombinant PsaA (“rPsaA”) from S. pneumoniae and capsular polysaccharide from N. meningitidis serogroup C. This disclosure further relates to methods for producing the immunogenic composition as well as methods for their use.
Owner:HOWARD UNIVERSITY +1
Fusion gene and application in preparation of pneumococcal vaccines
ActiveCN107828810AImproving immunogenicityHas immune effectAntibacterial agentsBacterial antigen ingredientsAntigenVaccine Immunogenicity
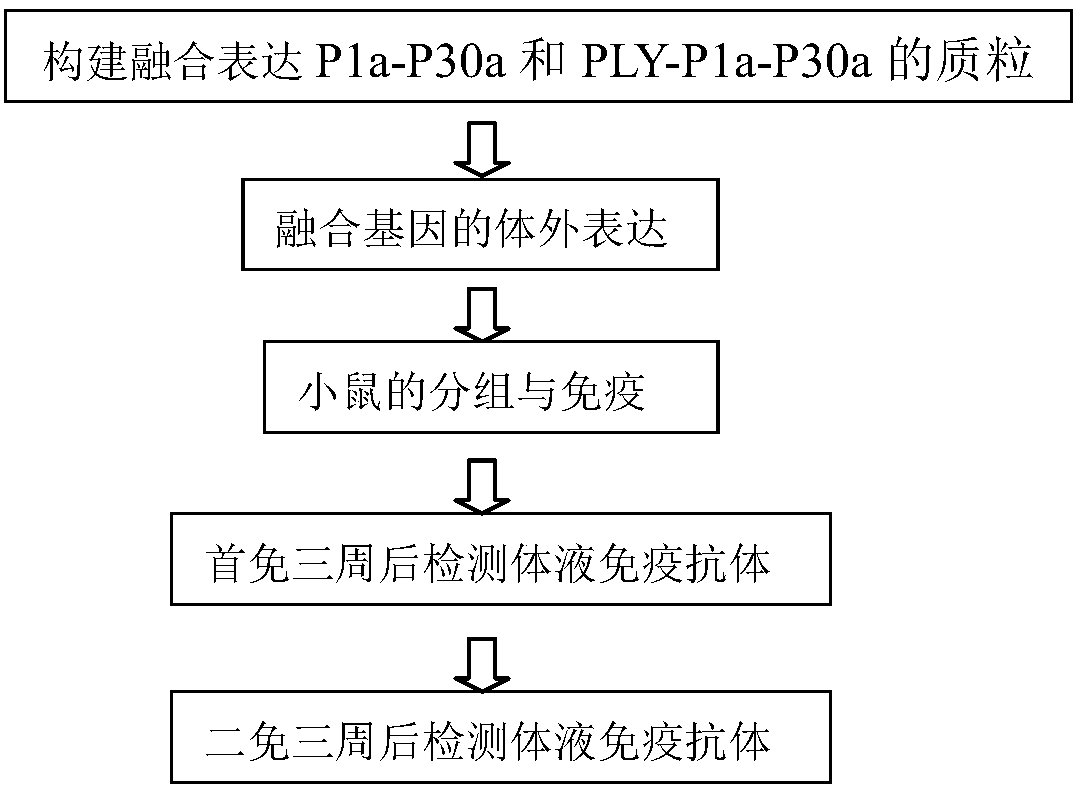
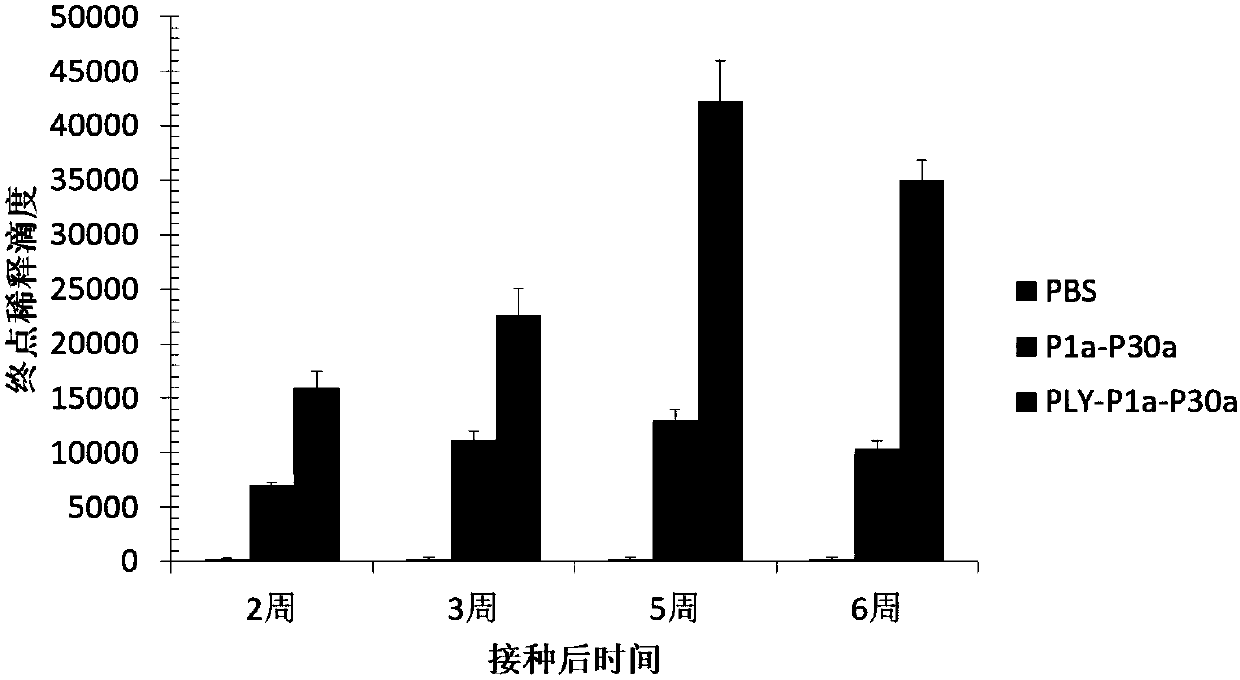
The invention relates to a fusion gene. The fusion gene is characterized in that a dominant antigen area P1a-P30a of mycoplasma pneumoniae P1 and P30 is subject to fusion expression; the PLY-P1a-P30ais subject to fusion expression by a gene of pneumolysin PLY and one part of genes of mycoplasma pneumoniae P1 and P30; the immunogenicity of the protein PLY-P1a-P30a is obviously better than the immunogenicity of P1a-P30a; the immunogenicity of the mycoplasma pneumoniae subunit P1a-P30a is enhanced; the immunity function of streptococcus pneumoniae is realized, and the toxicity of mouse is not observed in clinical application; the fusion gene PLY-P1a-P30a is a gene resource with lower cost, the expression quantity of subunit vaccine of the fusion gene PLY-P1a-P30a is high, and the purification and preparation are easy; the stability and immune activity can be maintained for two years or more at the temperature of -80 DEG C; compared with the conventional deactivating vaccine and weak-toxic vaccine, the preparation cost is obviously reduced, and the foundation is laid for the study of mycoplasma pneumoniae and streptococcus pneumoniae vaccines.
Owner:嘉兴迈维代谢生物科技有限公司
Novel pneumococcal vaccine formulations
ActiveUS20190307873A1Improve protectionEasy to adaptAntibacterial agentsBacterial antigen ingredientsStreptococcus mitisImmunogenicity
Immunogenic composition are provided comprising PnCo and / or GlpO polypeptides identified as being preferentially expressed during the virulent phase of an infection related to streptococcal bacteria. The compositions can be used for eliciting immune response against streptococcal infections, such as against infections caused by S. pneumoniae.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method for preparing pneumococcal capsular polysaccharide and pneumococcal vaccine
PendingCN114853917AGood purification effectHigh purityAntibacterial agentsBacterial antigen ingredientsTGE VACCINEPneumococcal vaccine
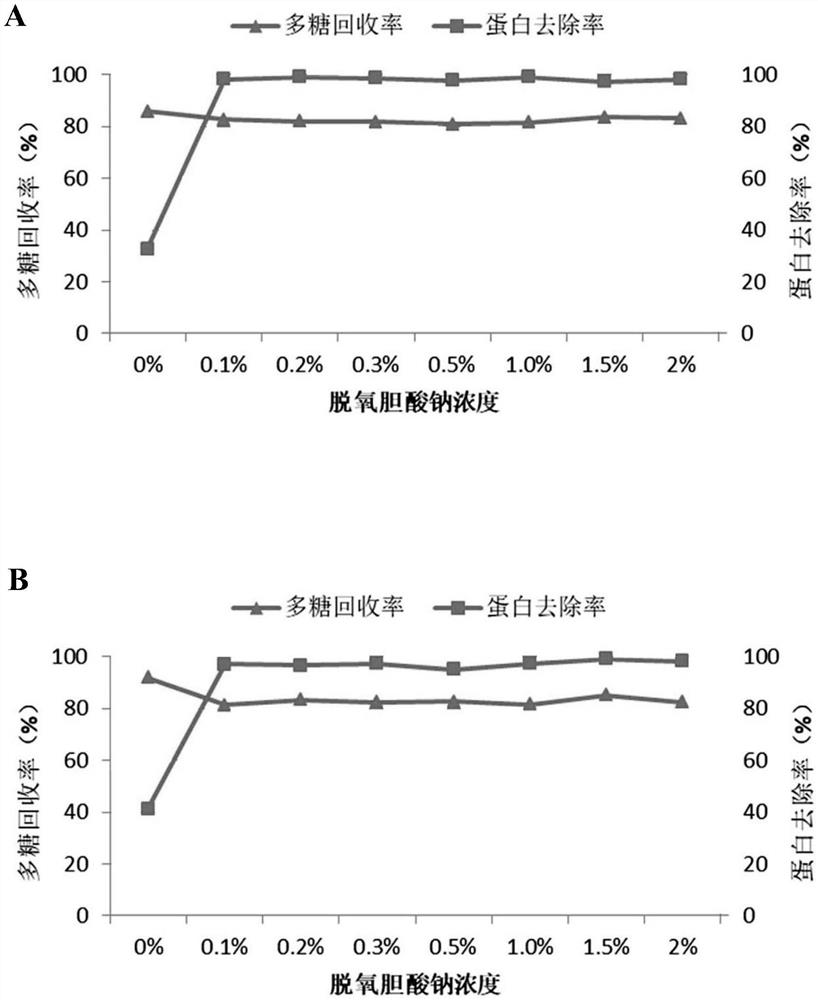
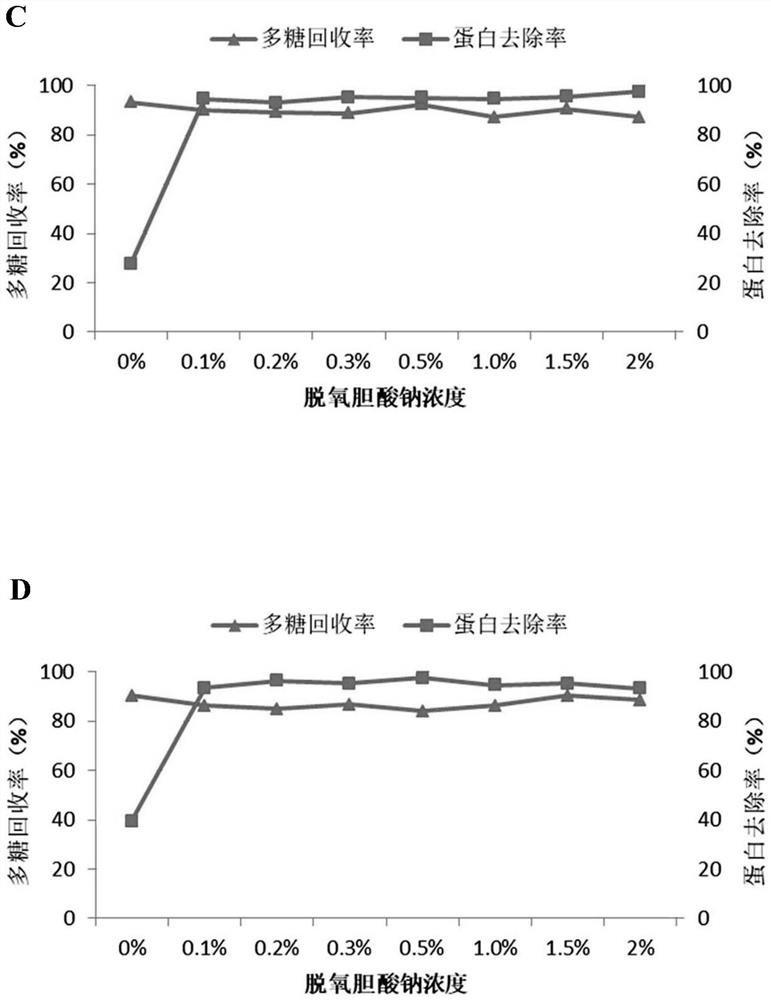
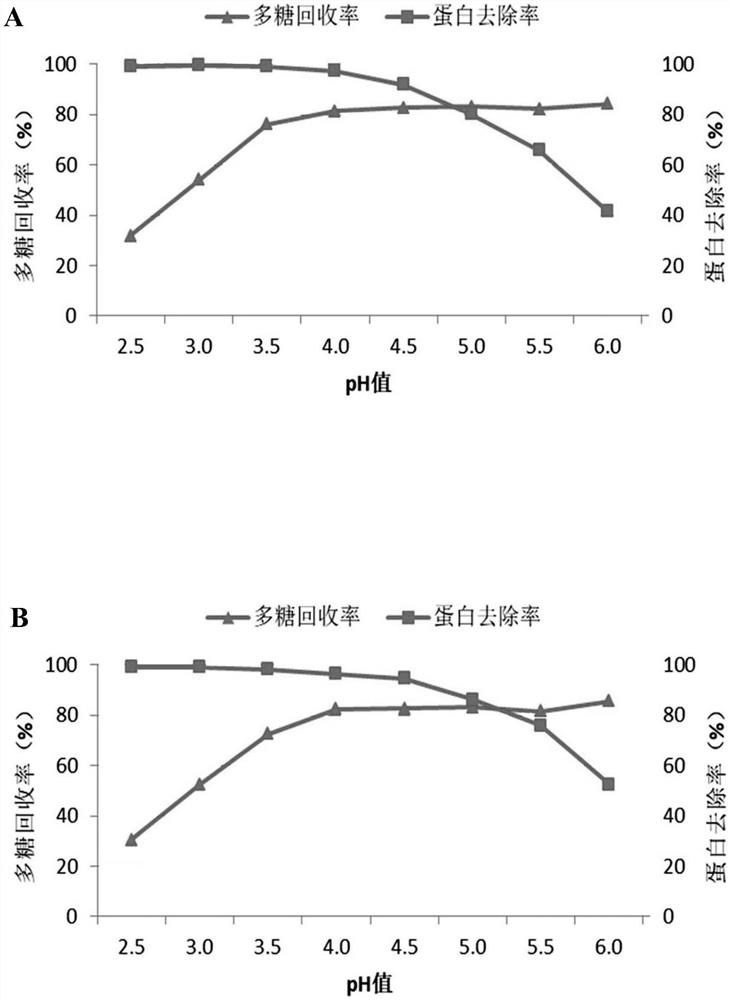
The invention relates to a method for preparing pneumococcal capsular polysaccharide and a pneumococcal vaccine. A method of making a pneumococcal capsular polysaccharide comprises providing a pneumococcal lysate of a selected serotype; removing proteins and nucleic acids from the lysate, the removal of proteins and the removal of nucleic acids being performed out of order in separate steps; the method for removing the protein comprises the following steps: I) mixing a sample to be treated with sodium deoxycholate to enable the pH value of the mixed system to be 3-6, centrifuging to obtain supernate, and adjusting the pH value of the supernate to be 6-8 to obtain first supernate; iI) removing sodium deoxycholate in the first supernate; the method for removing the nucleic acid comprises the following steps: A) mixing a sample to be treated with divalent salt to enable the pH value of the mixed system to be 1-4, centrifuging to obtain supernate, and adjusting the pH value of the supernate to be 6-8 to obtain second supernate; and B) removing the divalent salt added in the step A) in the second supernatant. The method is simple and ingenious, and an excellent pneumococcus capsular polysaccharide purification effect is achieved.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Pneumococcal vaccine formulations
ActiveUS11103568B2Easy to adaptAntibacterial agentsBacterial antigen ingredientsPneumococcal vaccineImmunogenicity
Immunogenic composition are provided comprising PnCo and / or GlpO polypeptides identified as being preferentially expressed during the virulent phase of an infection related to streptococcal bacteria. The compositions can be used for eliciting immune response against streptococcal infections, such as against infections caused by S. pneumoniae.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Vaccine for preventing pneumococus infection
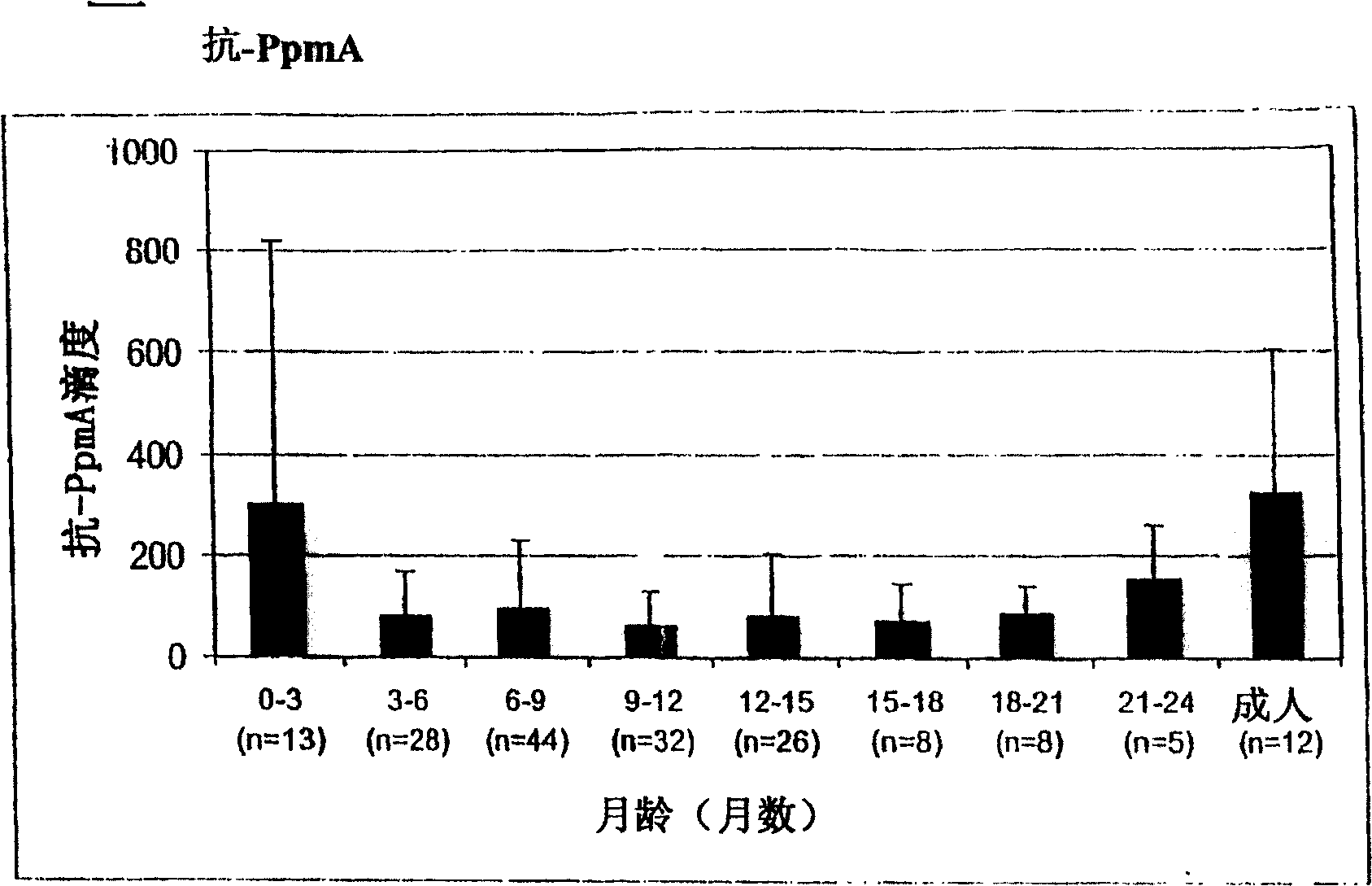
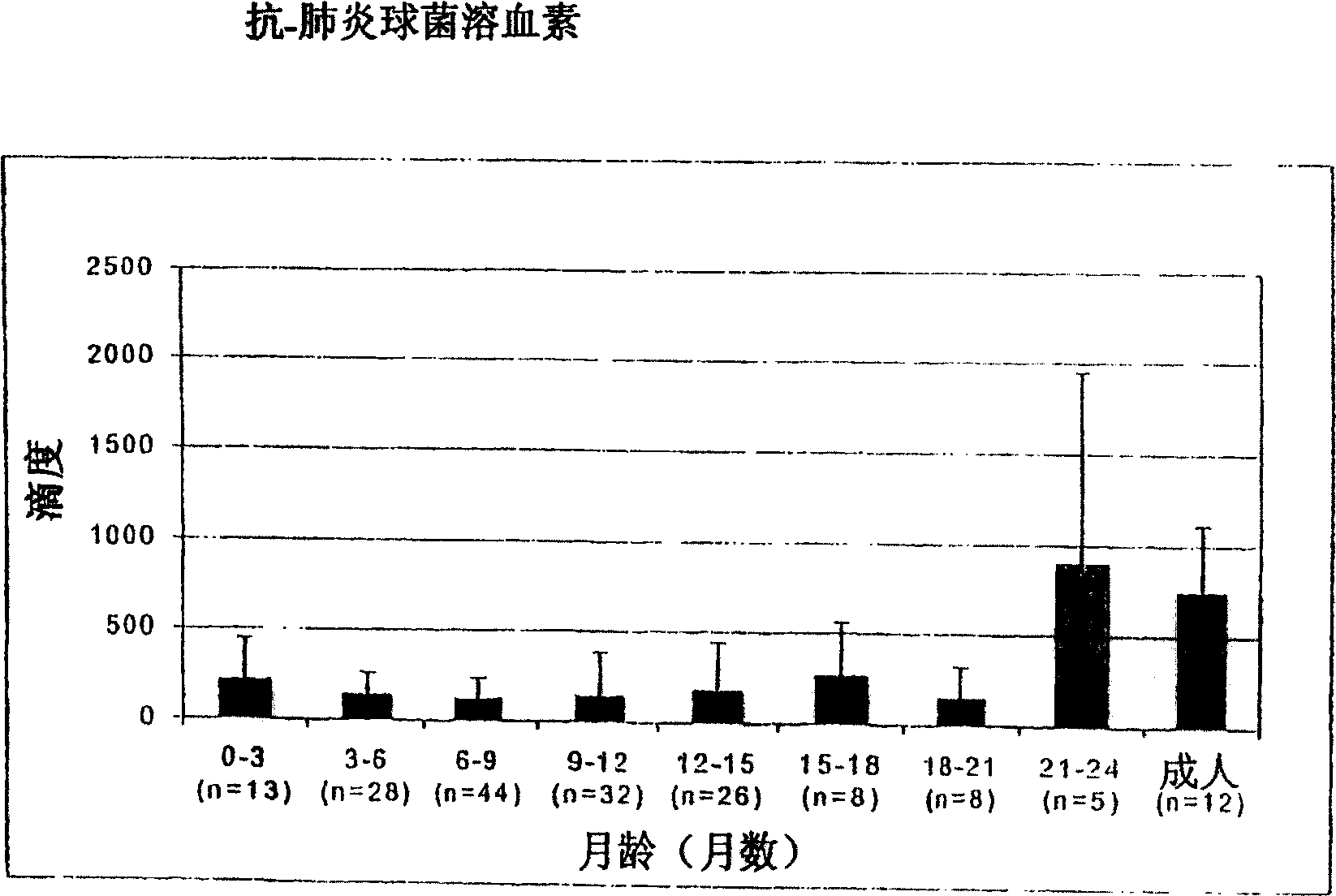
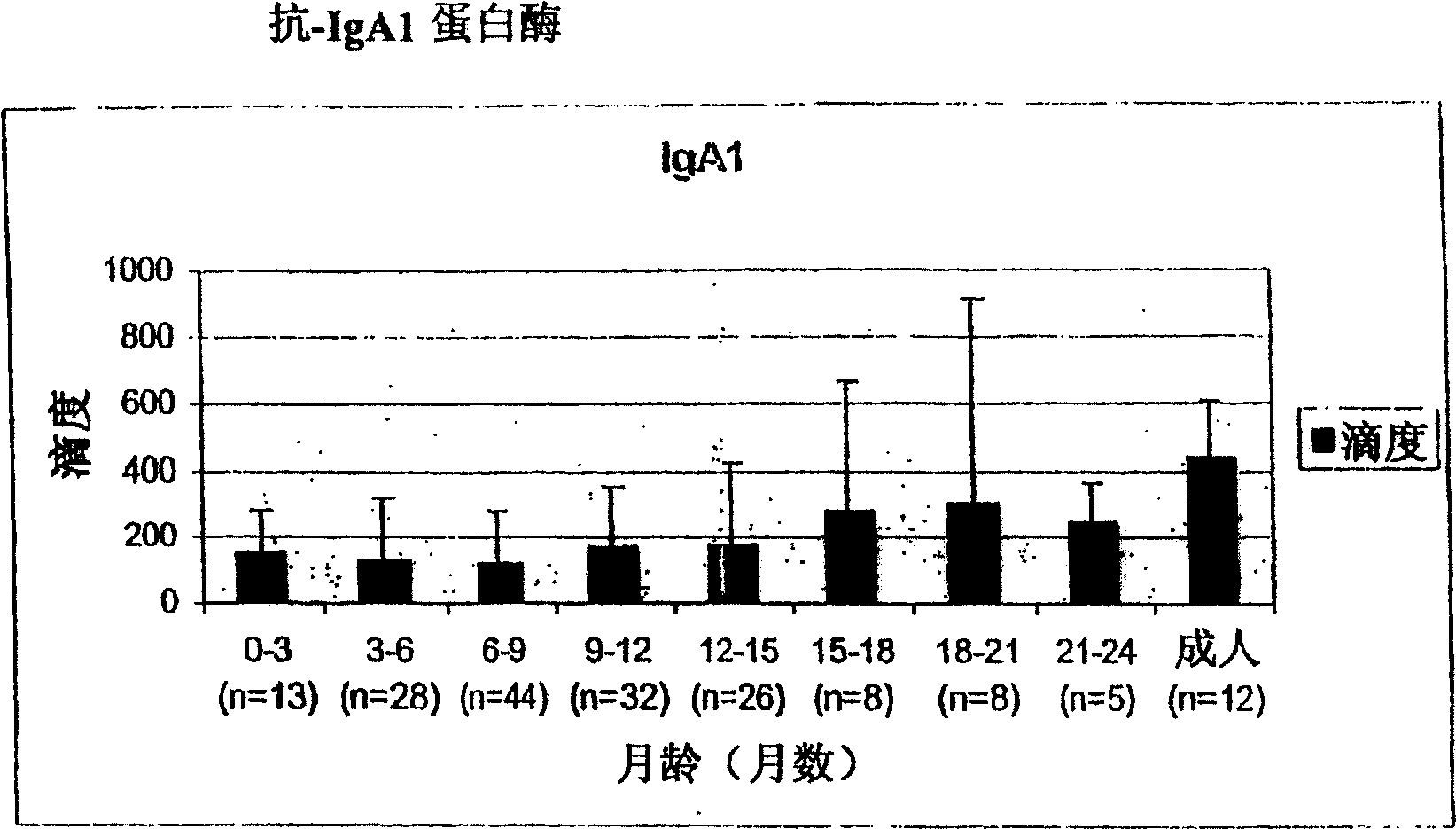
The invention relates to the field of biology, more specifically to the field of immunology and microbiology. The invention further relates to the field of vaccines against microbial infections and especially bacterial vaccines, in particular to pneumococcal vaccines. More in particular, the invention relates to means and methods to identify, select and isolate a vaccine component for passive and / or active immunisation against a microorganism that can be killed by opsonophagocytic cells. <??>The invention relates to a method to identify an opsonophagocytosis inducing antigen as a vaccine component for immunisation against a microorganism. The invention describes three pneumococcal proteins SlrA, IgA1 proteinase, and PsaA, and their use as a vaccine component with or without PpmA. <??>The invention also discloses the use of antibodies against said proteins for passive immunisation.
Owner:MUCOSIS
a pneumococcal vaccine
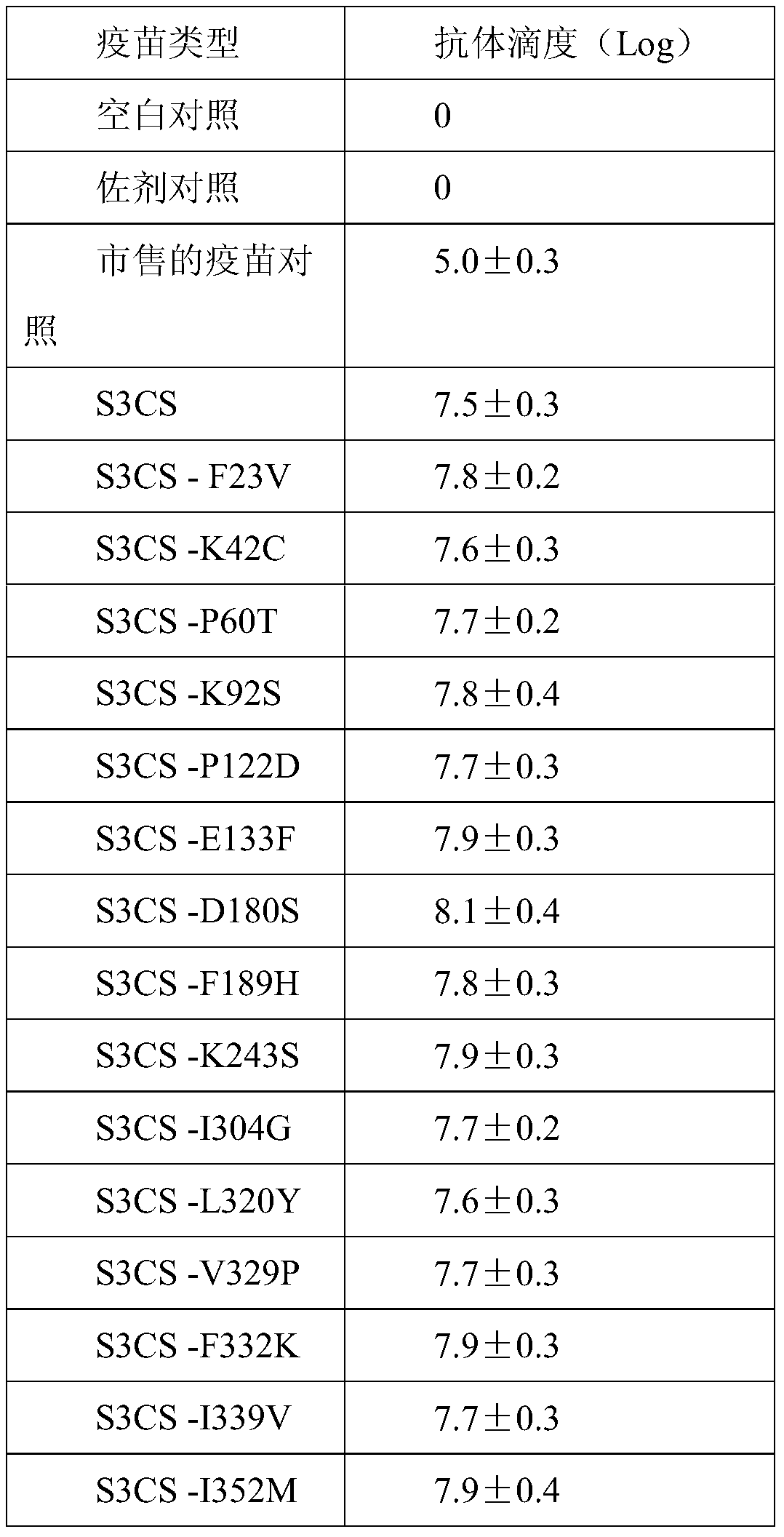
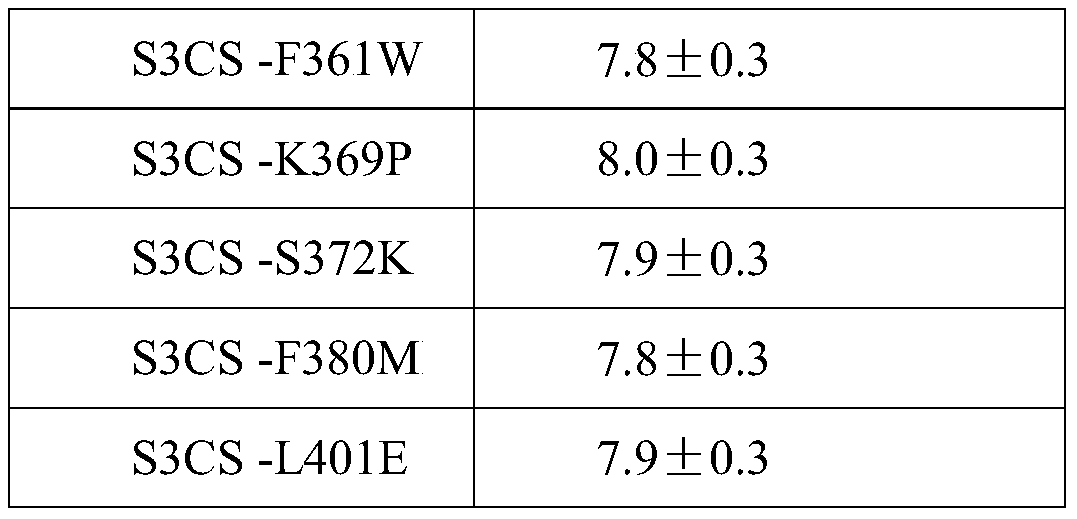
ActiveCN105963691BImproving immunogenicityGood immune protectionAntibacterial agentsBacterial antigen ingredientsAdjuvantMutated protein
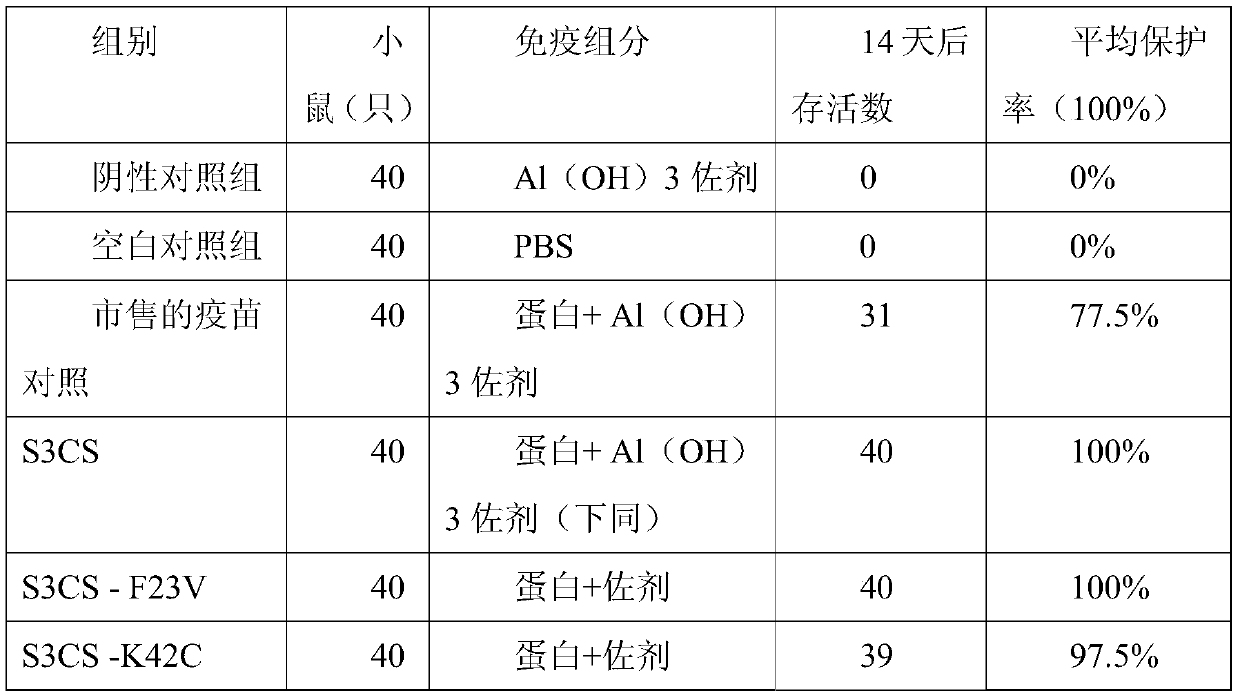
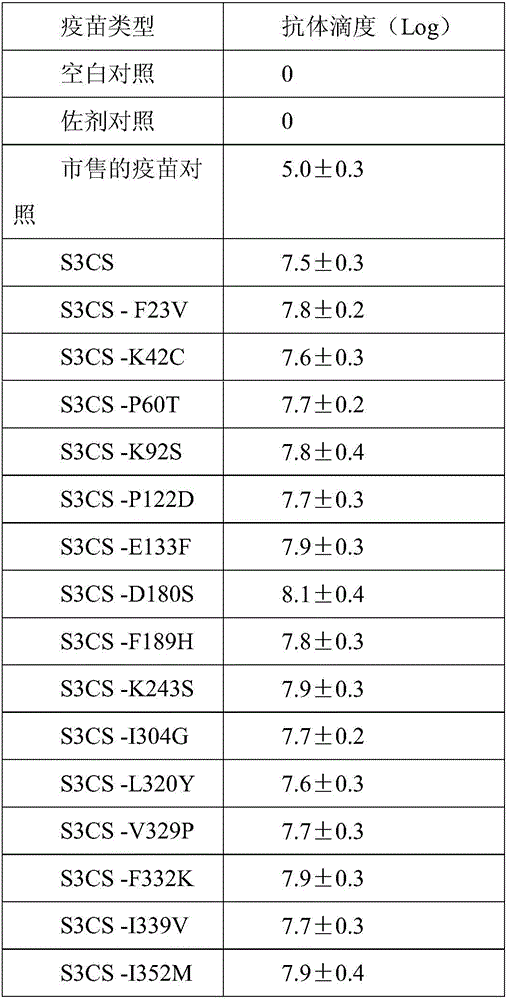
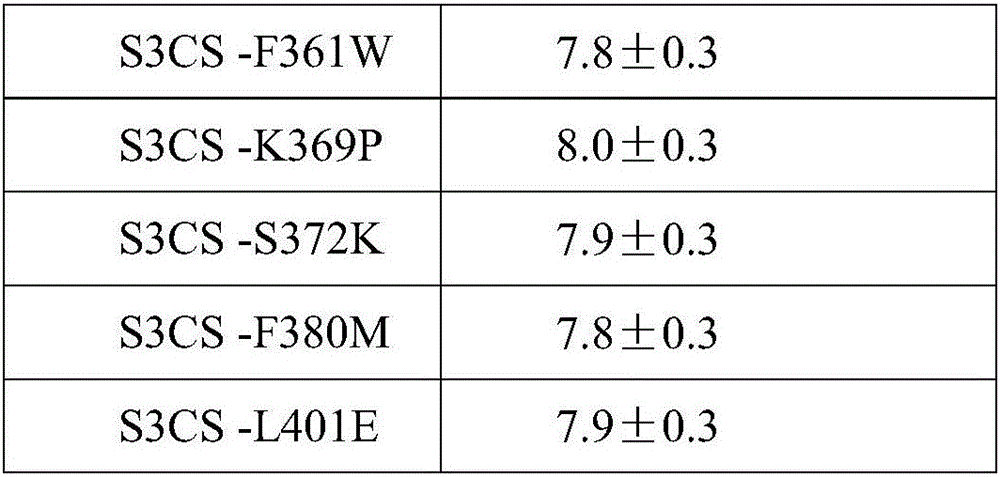
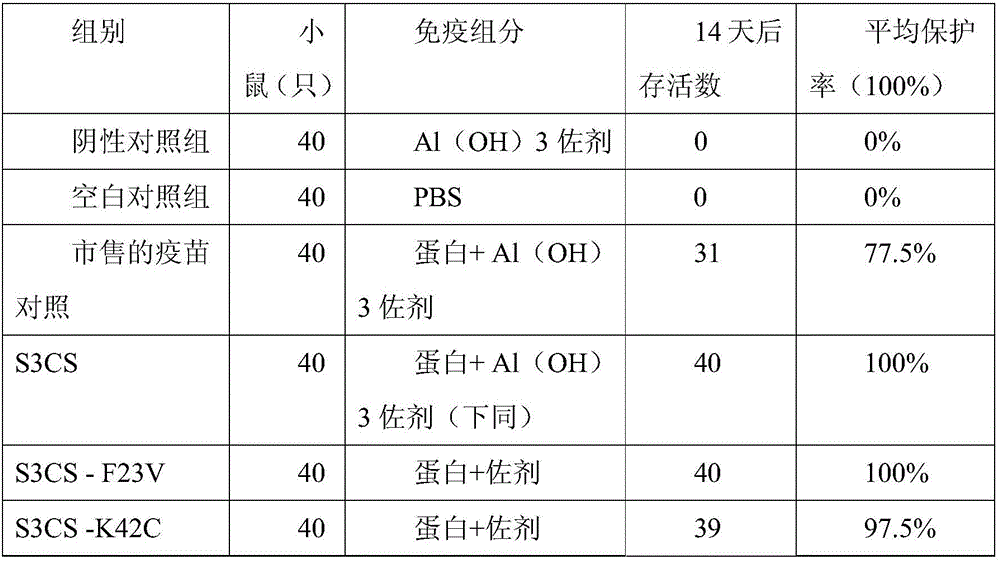
The invention discloses a streptococcus pneumoniae vaccine and a preparation method thereof. The streptococcus pneumoniae vaccine is prepared by mixing S3CS recombinant antigens or mutant protein antigens with additives. The vaccine can be applied to immunity; the protection of organisms on streptococcus pneumonia can be improved; a specific antibody can be generated. A preparation method of the vaccine is simple; the requirements of large-scale industrial production can be met.
Owner:SINO UNITED (BEIJING) BIOMEDICAL TECH CO LTD
Streptococcus pneumoniae vaccine
ActiveCN105963691AImproving immunogenicityGood immune protectionAntibacterial agentsBacterial antigen ingredientsAdjuvantMutated protein
The invention discloses a streptococcus pneumoniae vaccine and a preparation method thereof. The streptococcus pneumoniae vaccine is prepared by mixing S3CS recombinant antigens or mutant protein antigens with additives. The vaccine can be applied to immunity; the protection of organisms on streptococcus pneumonia can be improved; a specific antibody can be generated. A preparation method of the vaccine is simple; the requirements of large-scale industrial production can be met.
Owner:诺赛联合(北京)生物医学科技有限公司
Immunogenic compositions for use in pneumococcal vaccines
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 18, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
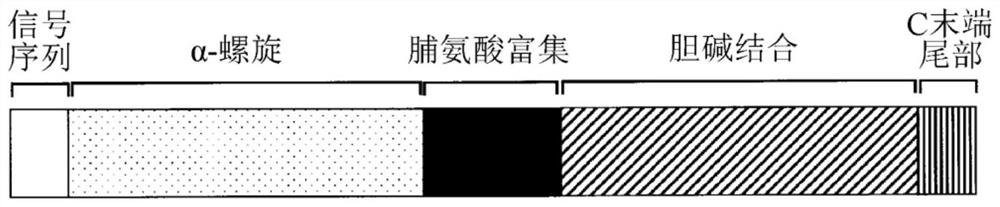
Pneumococcal surface proteins
The present invention provides D39-derived mutant PspA that does not undergo deamination and maintains molecular stability even at near-neutral pH. Specifically, the present invention is the following protein (a) or (b). (a) A protein that includes the amino acid sequence represented by sequence no. 2 and that has pneumococcus vaccine antigenic activity, and a protein substantially the same as said protein; and (b) a protein that includes a portion of the amino acid sequence represented by sequence no. 2, said portion including aspartic acid at position 254, and that has pneumococcus vaccine antigenic activity, and a protein substantially the same as said protein.
Owner:THE UNIV OF TOKYO +1
Pneumococcal serotype 6d
InactiveUS20100209457A1Antibacterial agentsBacterial antigen ingredientsSynechococcusPneumococcal serotypes
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6D, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit→2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→4) ribitol (5→phosphate. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com