Streptococcus pneumoniae vaccine
A Streptococcus pneumoniae and vaccine technology, applied in the field of vaccines, can solve the problems of short-term immune response, low-affinity antibodies, and difficulty in immune enhancement, and achieve the effects of low cost, simple preparation, and good immune protection ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Expression of Example 1 Protein
[0036]Using the DNA of Streptococcus pneumoniae as a template, the gene sequence of the amino acid sequence shown in SEQ ID NO: 1 was amplified by designing primers, and then the PCR was extracted from the agar gel using the QIAquick gel extraction kit of QIAgen (Chatworth, CA). Product recovery. After digestion with the Superlinker carrier pSL301 (Invitrogen, San Diego, CA), the QIAquick gel extraction kit of QIAgen (Chatworth, CA) was used to recover from the agar gel. The genomic DNA cut by the restriction enzyme The fragment was ligated with the pSL301 vector cut with restriction enzymes. The ligated product was then transformed into DH5a Escherichia coli according to the method of Simanis (Hanahan, D.DNA Cloning, 1985, D.M.Glover (ed), pp.109-13 5 Middle. The pSL301 recombinant plasmid (rpSL301) containing the target gene was purified with a QIAgen kit, and after sequencing, it was confirmed that the recombination was correct.
[...
Embodiment 2
[0038] The acquisition of embodiment 2 mutein
[0039] Introduction of mutation point
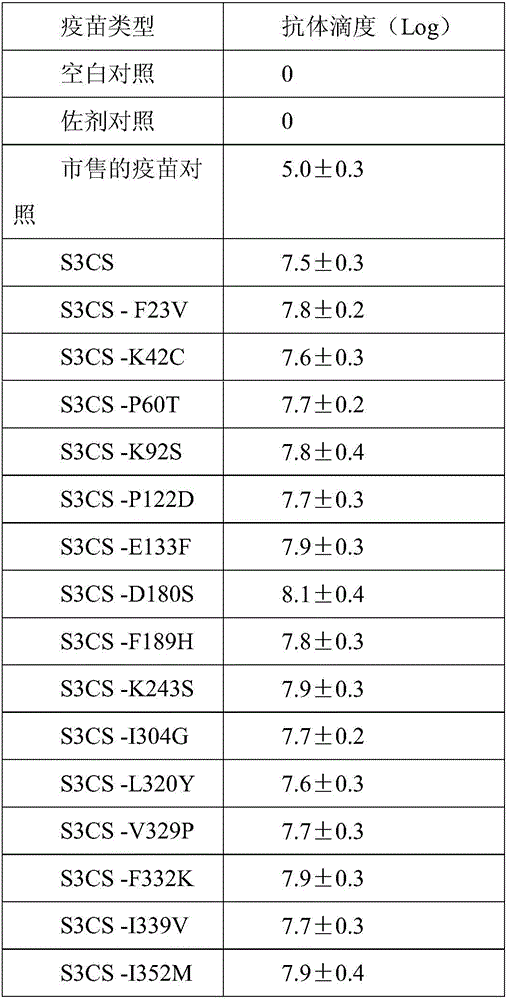
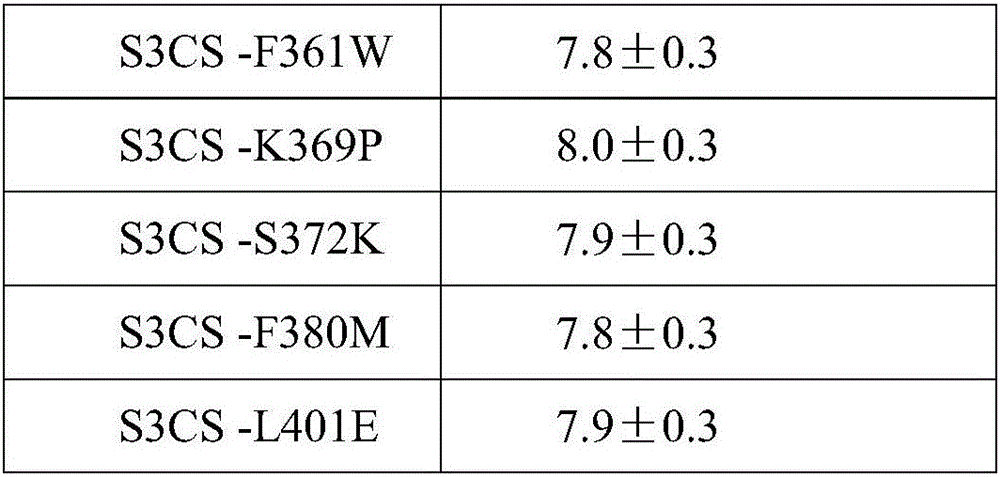
[0040] (1), design corresponding mutagenesis primers according to corresponding mutation sites, adopt PCR site-directed mutagenesis method to mutate the amino acid sites corresponding to the wild type on the corresponding S3CS gene; described mutation sites are in SEQ ID NO: 1 based on point mutations. F23V (indicates that the F amino acid at position 23 is replaced by V), K42C, P60T, K92S, P122D, E133F, D180S, F189H, K243S, I304G, L320Y, V329P, F332K, I339V, I352M, F361W, K369P, S372K, F380M , L401E.
[0041] (2), after the above-mentioned PCR product is recovered and purified by gel, it is digested with a restriction endonuclease, and after being ligated with the plasmid pSL301 carrier fragment through the same digestion, it is transformed into Escherichia coli DH5α competent cells;
[0042] (3) Identification of the recombinant plasmid: identification of the recombinant plasmid by enz...
Embodiment 3
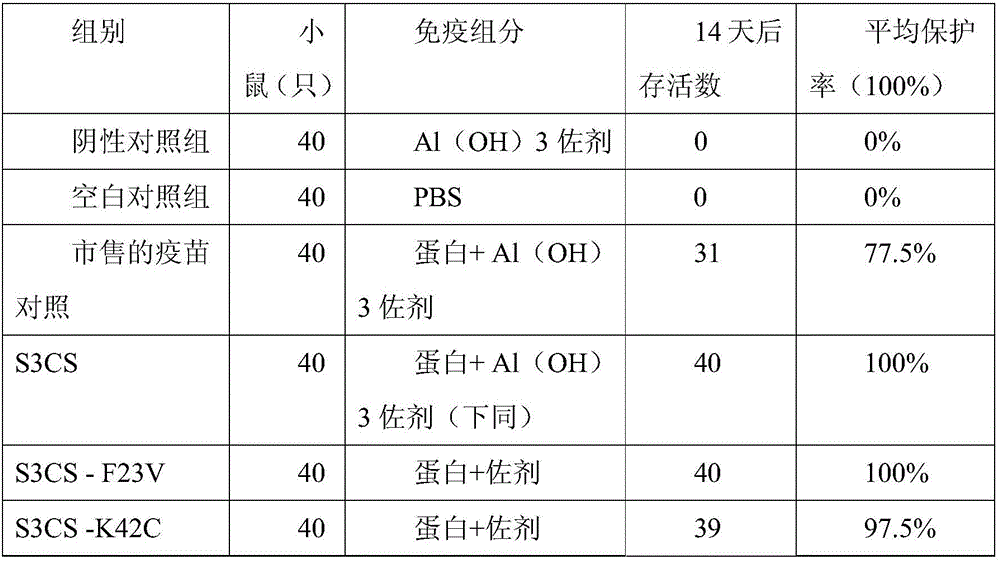
[0044] Example 3 Antibody Effect Evaluation of Pneumococcal Protein Vaccine
[0045] 12-14g female 3-week-old BALB / c. young mice were randomly divided into 23 groups, 20 in each group, and were injected intraperitoneally with the conjugated vaccine prepared according to the proteins of Examples 1 and 2 and Al(OH)3 as an immune adjuvant, Commercially available 13-valent lung chain conjugate vaccine, blank control (PBS).
[0046] On the 0th, 2nd, and 4th weeks, the program injected 3 times, and each young mouse contained 0.5ml (0.5ug protein). Blood was collected from each experimental group 7 days after the third injection, the serum was separated, and stored at -20°C for future use.
[0047] For the detection of protein antibody titer, the indirect ELISA method was used, and the protein was dissolved in 0.05mol / L pH9.6 carbonate buffer solution, coated with 20ug / ml concentration on the microtiter plate, and blocked with 2% BSA solution. During the experiment, the serum to be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com