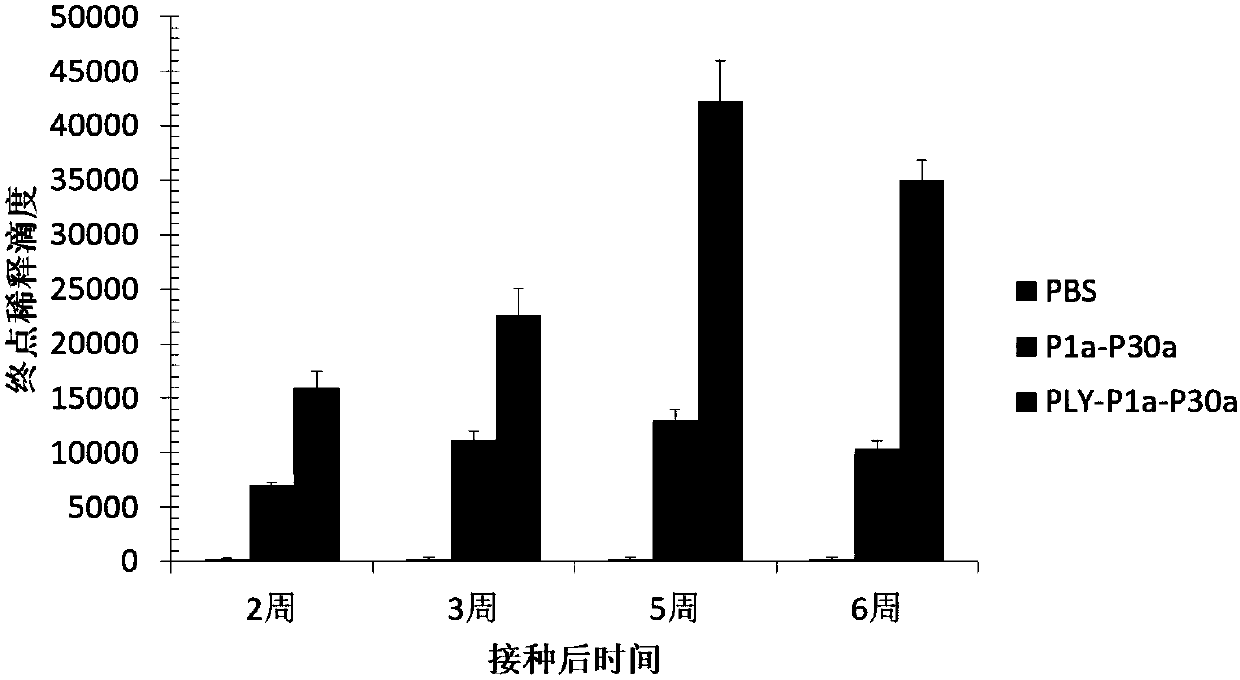
Fusion gene and application in preparation of pneumococcal vaccines
A fusion gene and gene technology, applied in the field of medicine and biology, can solve the problems of poor immunogenicity and no retention of pathogenic particles, etc., achieve high expression, easy purification and preparation, and enhance the effect of immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Construction of pET30a-P1a-Linker-P30a fusion gene
[0026] The P1a-linker-P30 sequence uses the Mycoplasma pneumoniae M129 strain as the sequence template. P1a and P30a respectively select the 3478-4047 bases of the M129 strain P1 and the 311-792 bases of the P30, and connect them through Linker. The company synthesized it on the PUC19-P1a-Linker-P30a vector, cut it with NcoI and XhoI, and connected it to the pET30a vector.
[0027] The PUC19-P1a-Linker-P30a enzyme digestion system is as follows:
[0028]
[0029] The enzyme digestion system of pET30a vector is as follows:
[0030]
[0031] The pUC19-P1a-Linker-P30a enzyme digestion system and the pET30a vector enzyme digestion system were reacted at 37°C for 2 hours, the digestion results were detected by 1% agarose gel electrophoresis, and the target fragment was purified and recovered. Ligate the vector and target fragments with T4 ligase,
[0032] Its reaction system is as follows:
[0033] ca...
Embodiment example 2
[0044] Example 2: Prokaryotic expression of recombinant plasmid pET30a-P1a-Linker-P30a
[0045] Plasmid Transformation The expression plasmid pET30a-P1a-Linker-P30a (Kana-resistant) of the P1a-P30a fusion gene was transformed into Escherichia coli BL21 competent cells.
[0046] The specific operation is as follows:
[0047] 1) Transfer 50 μL of Escherichia coli BL21 competent cell suspension to a sterile 1.5ml EP tube, add 1 μL of the ligation product, swirl gently to mix the contents, and place on ice for 30 minutes.
[0048] 2) Heat shock in a water bath at 42°C for 90s (let the plasmid enter competent cells).
[0049] 3) Take it out and place it on ice for 5 minutes (let the competent cells close).
[0050] 4) Add 200 μL LB medium to each tube. Warm the medium to 37°C with a water bath, then transfer the centrifuge tube to a shaker at 37°C, and incubate for 60 minutes to recover the bacteria. In order to achieve effective conversion, the rotating speed is 180r / min.
[...
Embodiment example 3
[0053] Example 3: Prokaryotic expression of recombinant plasmid pET30a-PLY-Linker-P1a-Linker-P30a
[0054] Plasmid Transformation The expression plasmid pET30a-PLY-Linker-P1a-Linker-P30a (Kana-resistant) of the PLY-P1a-P30a fusion gene was transformed into Escherichia coli BL21 competent cells.
[0055] The specific operation is as follows:
[0056] 1) Transfer 50 μL of Escherichia coli BL21 competent cell suspension to a sterile 1.5ml EP tube, add 1 μL of the ligation product, swirl gently to mix the contents, and place on ice for 30 minutes.
[0057] 2) Heat shock in a water bath at 42°C for 90s (let the plasmid enter competent cells).
[0058] 3) Take it out and place it on ice for 5 minutes (let the competent cells close).
[0059] 4) Add 200 μL LB medium to each tube. Warm the medium to 37°C with a water bath, then transfer the centrifuge tube to a shaker at 37°C, and incubate for 60 minutes to recover the bacteria. In order to achieve effective conversion, the rotati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com