Method for preparing pneumococcal capsular polysaccharide and pneumococcal vaccine
A technology of pneumococcus and capsular polysaccharides, which is applied in the direction of antibacterial drugs and bacterial antigen components, can solve the problems of ethanol operation and storage safety hazards, extremely high requirements for production plants, and increase production costs, and achieve excellent purification effects and eliminate Potential safety hazards and the effect of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Purification of type 1 pneumococcal capsular polysaccharide
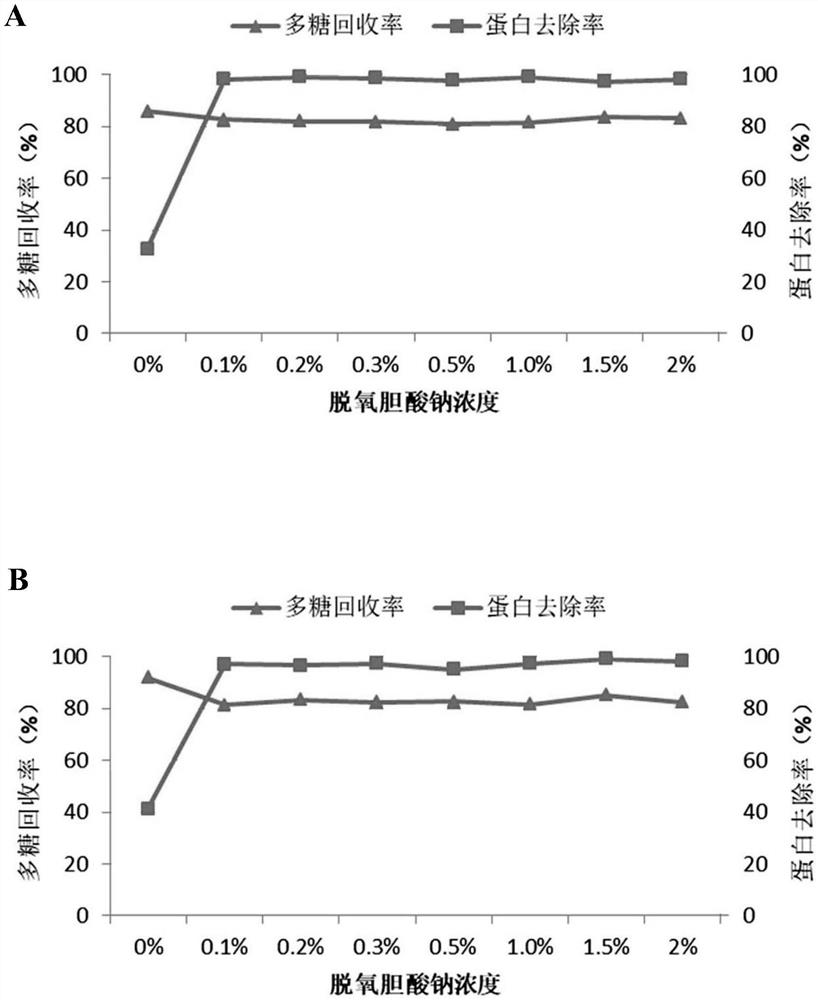
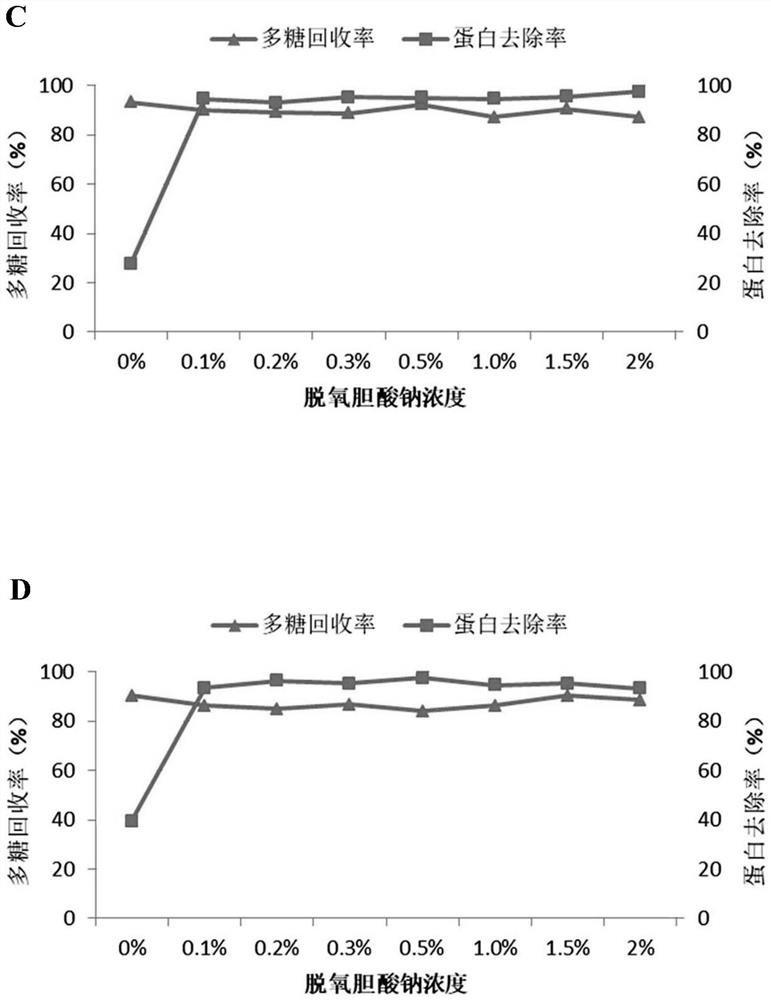
[0083] Type 1 pneumococcus was fermented in a bioreactor, and after growing to the logarithmic growth phase, sodium deoxycholate was added to lyse the bacteria. The supernatant was collected by centrifugation, and concentrated by ultrafiltration with an ultrafiltration membrane with a molecular weight cut-off of 300 Kd to obtain a type 1 pneumococcal lysate.
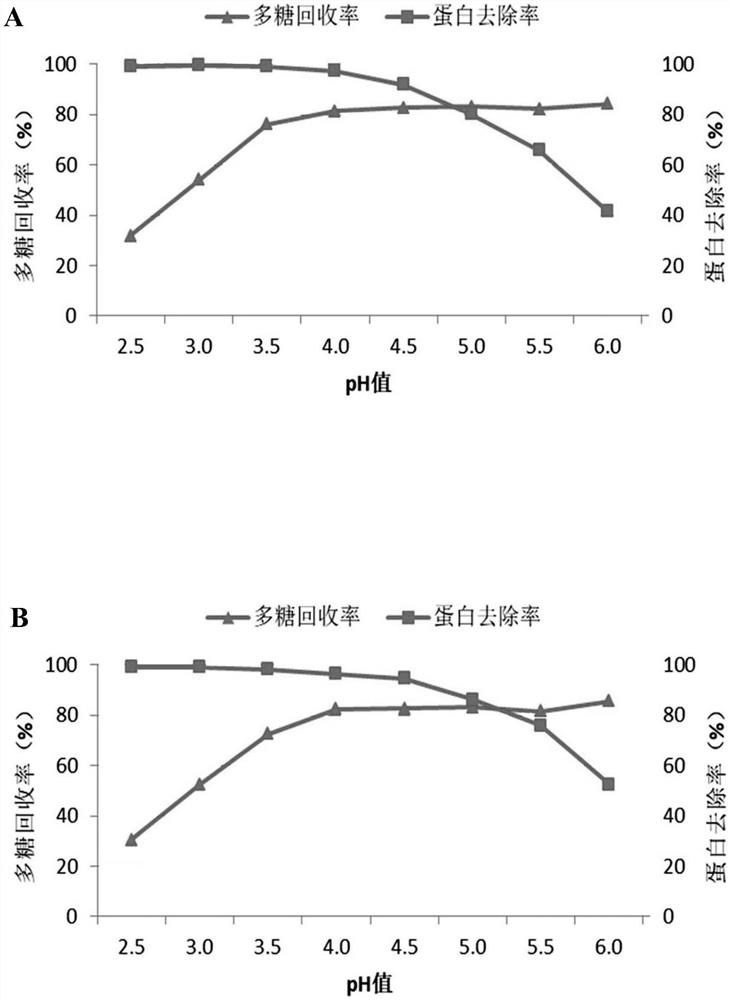
[0084] Sodium deoxycholate was added to the lysate of type 1 pneumococcus to make the final concentration 0.5%, the pH was adjusted to 4.5, left at room temperature for 10 minutes, centrifuged (centrifugal force: 6000 g, centrifugation time: 25 minutes), and the supernatant was collected , adjust the pH to 7.0, the solution is slightly cloudy at this time. Add sodium deoxycholate again to make the final concentration 0.5%, adjust the pH to 4.5, stand at room temperature for 10 minutes, centrifuge (centrifugal force: 6000g, centrifugation tim...
Embodiment 2
[0087] Example 2: Purification of type 1 pneumococcal capsular polysaccharide
[0088] Type 1 pneumococcal lysate was prepared as described in Example 1.
[0089] Calcium chloride was added to the lysate of type 1 pneumococcus to make the final concentration 0.2 mol / L, the pH was adjusted to 2.5, left standing at room temperature for 5 minutes, centrifuged (centrifugal force: 6000 g, centrifugation time: 25 minutes), and the supernatant was collected. solution, adjust the pH to 7.0. The supernatant was concentrated by ultrafiltration through a 300Kd membrane pack. Add sodium deoxycholate to the ultrafiltration concentrate to make the final concentration 0.5%, adjust the pH to 4.5, stand at room temperature for 10 minutes, centrifuge (centrifugal force: 6000g, centrifugation time: 25 minutes), collect the supernatant, The pH was adjusted to 7.0, at which point the solution was slightly hazy. Add sodium deoxycholate again to make the final concentration 0.5%, adjust the pH to...
Embodiment 3
[0092] Example 3: Purification of type 5 pneumococcal capsular polysaccharide
[0093] Type 5 pneumococcus was fermented in a bioreactor, and after growth to the logarithmic growth phase, sodium deoxycholate was added to lyse the bacteria. The supernatant was collected by centrifugation, and concentrated by ultrafiltration using an ultrafiltration membrane with a molecular weight cut-off of 100 Kd to obtain a type 5 pneumococcal lysate.
[0094] Sodium deoxycholate was added to the pneumococcal type 5 lysate to make the final concentration 1%, the pH was adjusted to 4.3, left standing at room temperature for 20 minutes, centrifuged (centrifugal force: 6000 g, centrifugation time: 20 minutes), and the supernatant was collected , adjust the pH to 7.0, the solution is slightly cloudy at this time. Add sodium deoxycholate again to make the final concentration 1%, adjust pH to 4.3, stand at room temperature for 20 minutes, centrifuge (centrifugal force: 6000g, centrifugation time:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com