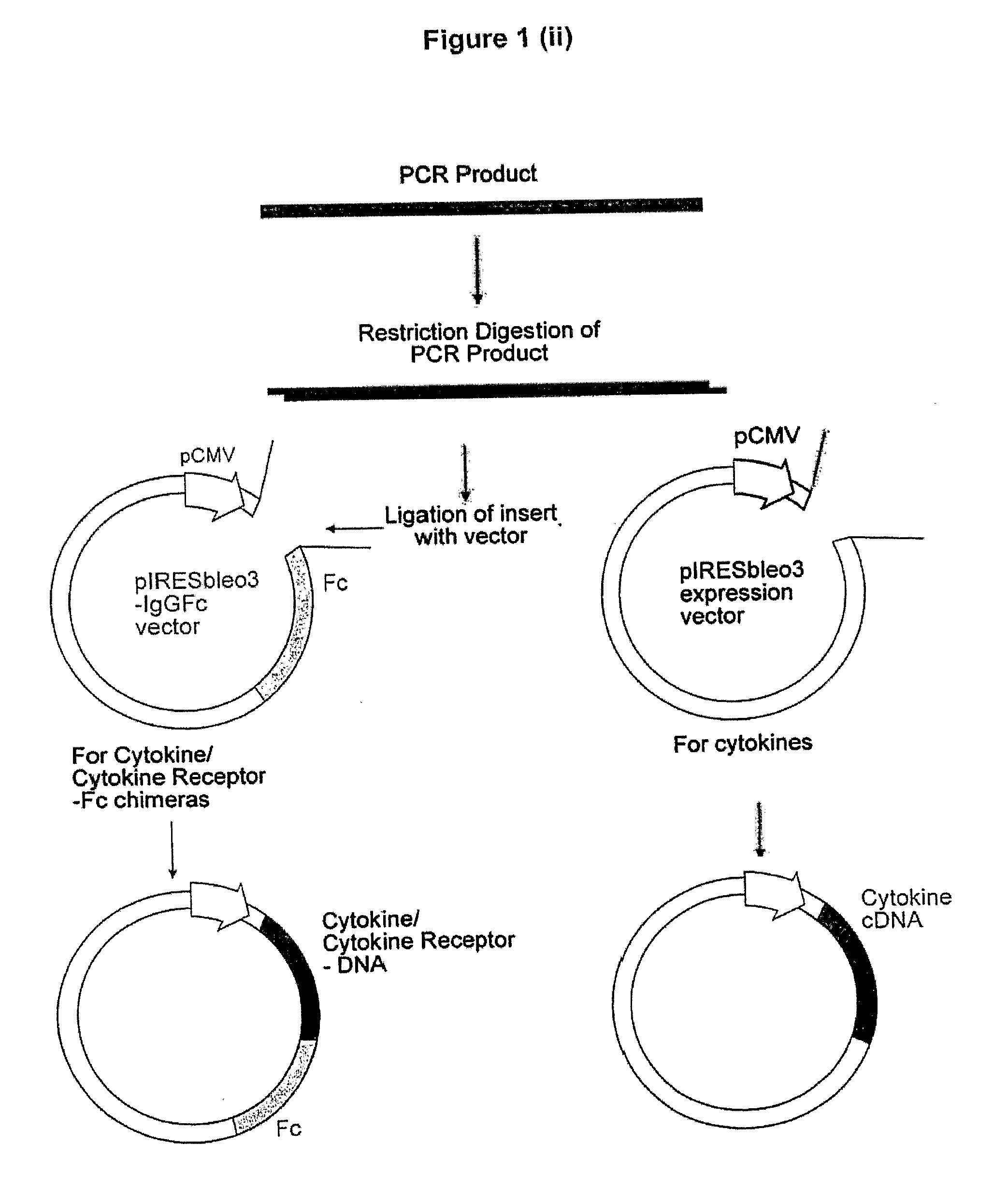
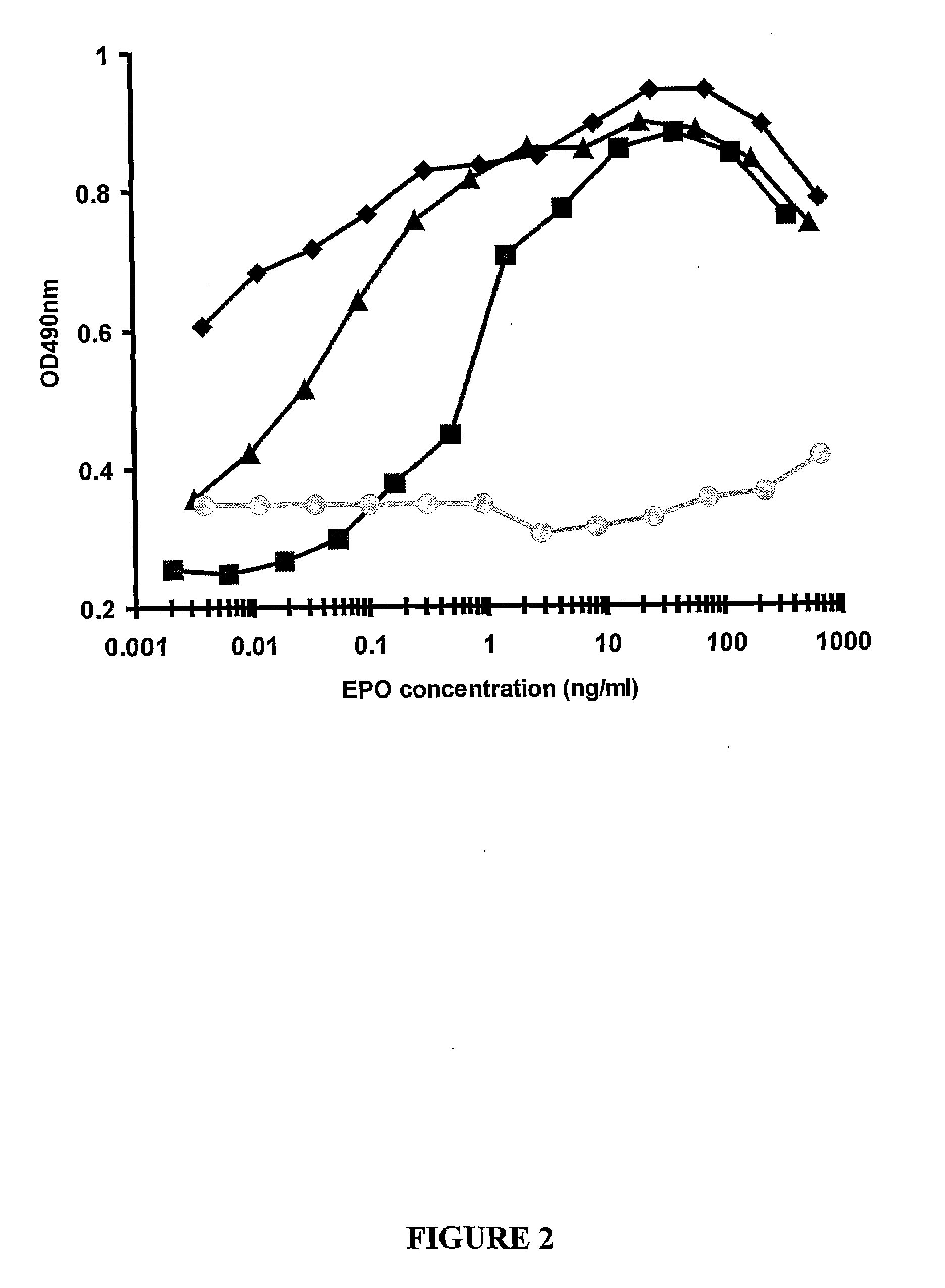
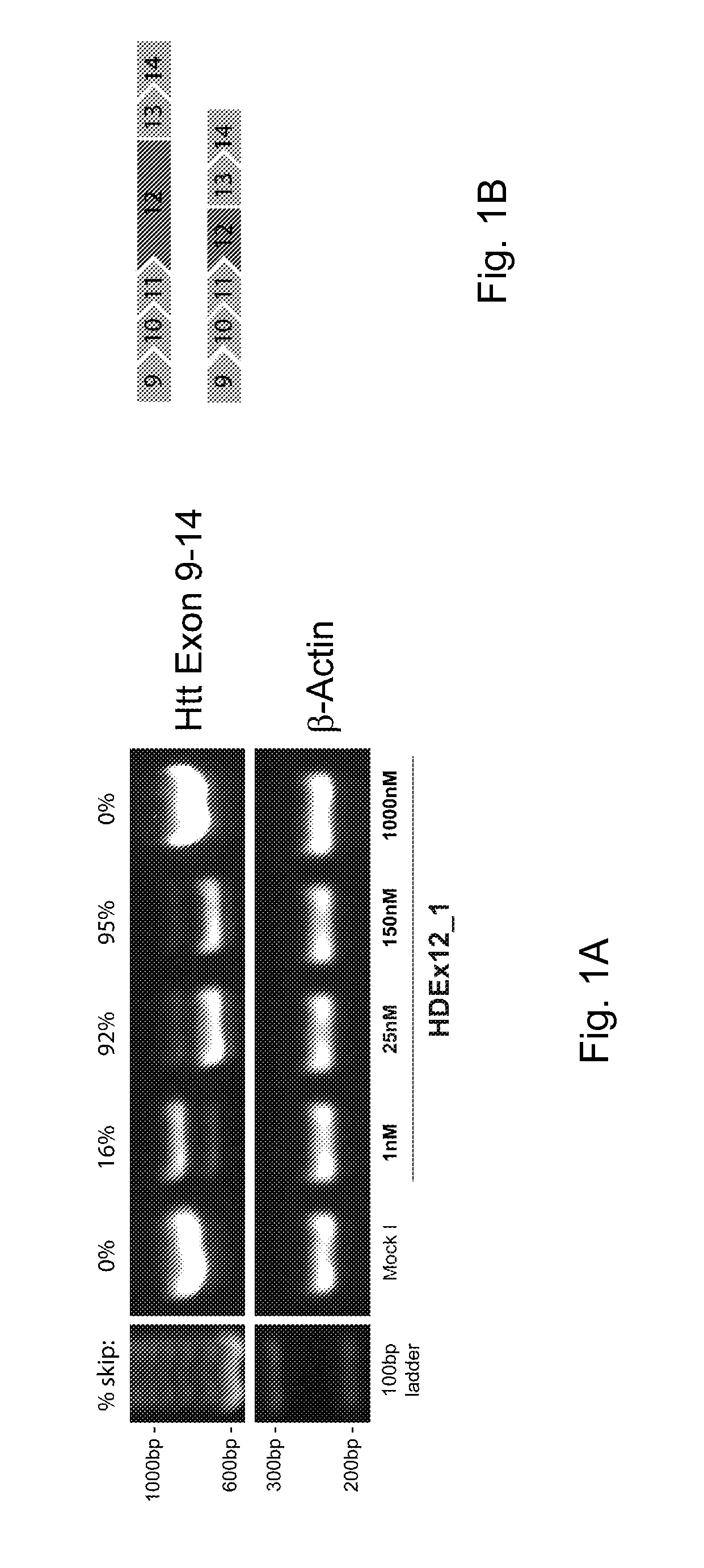
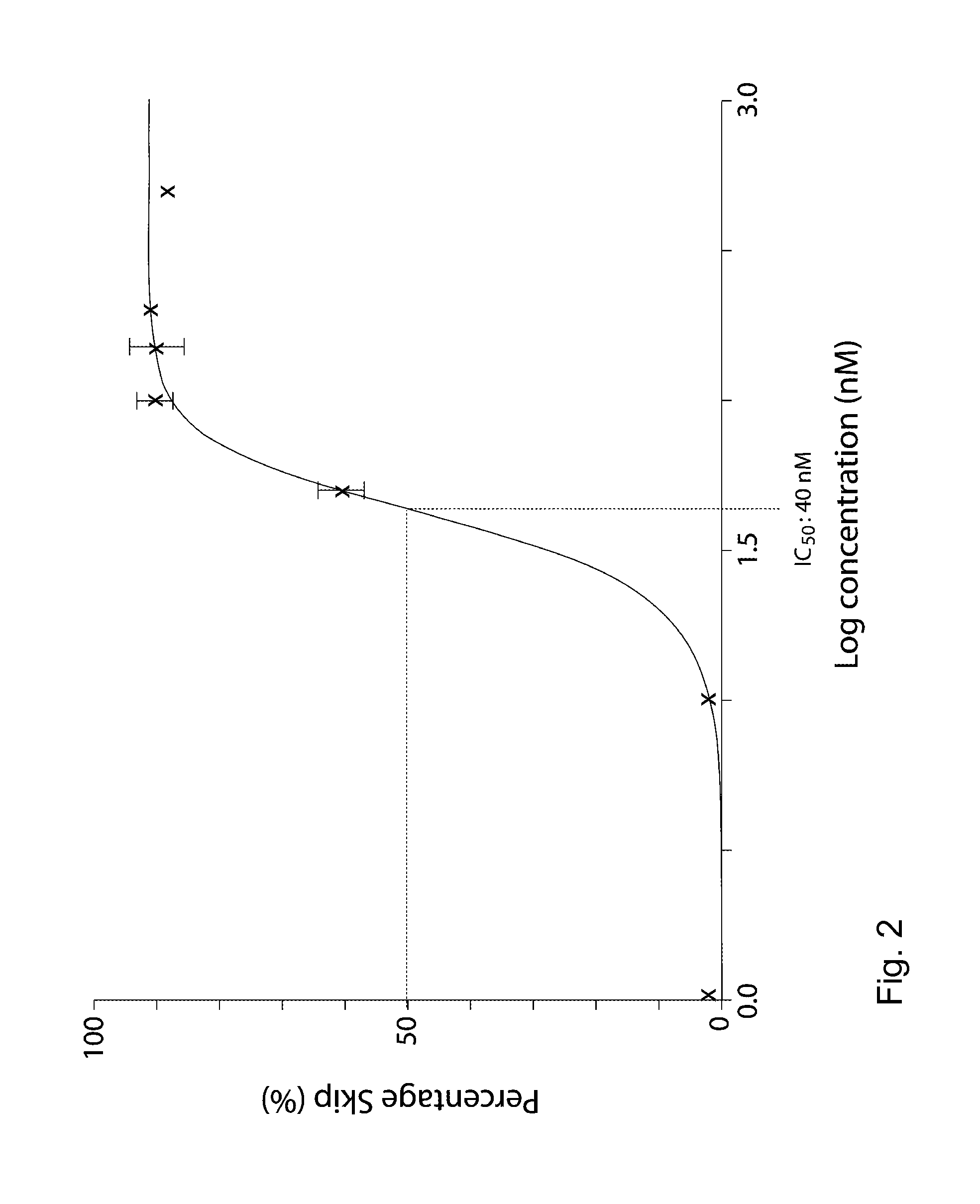
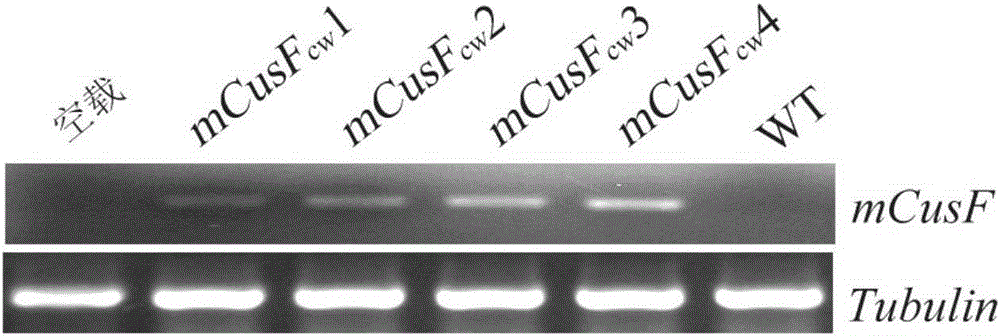
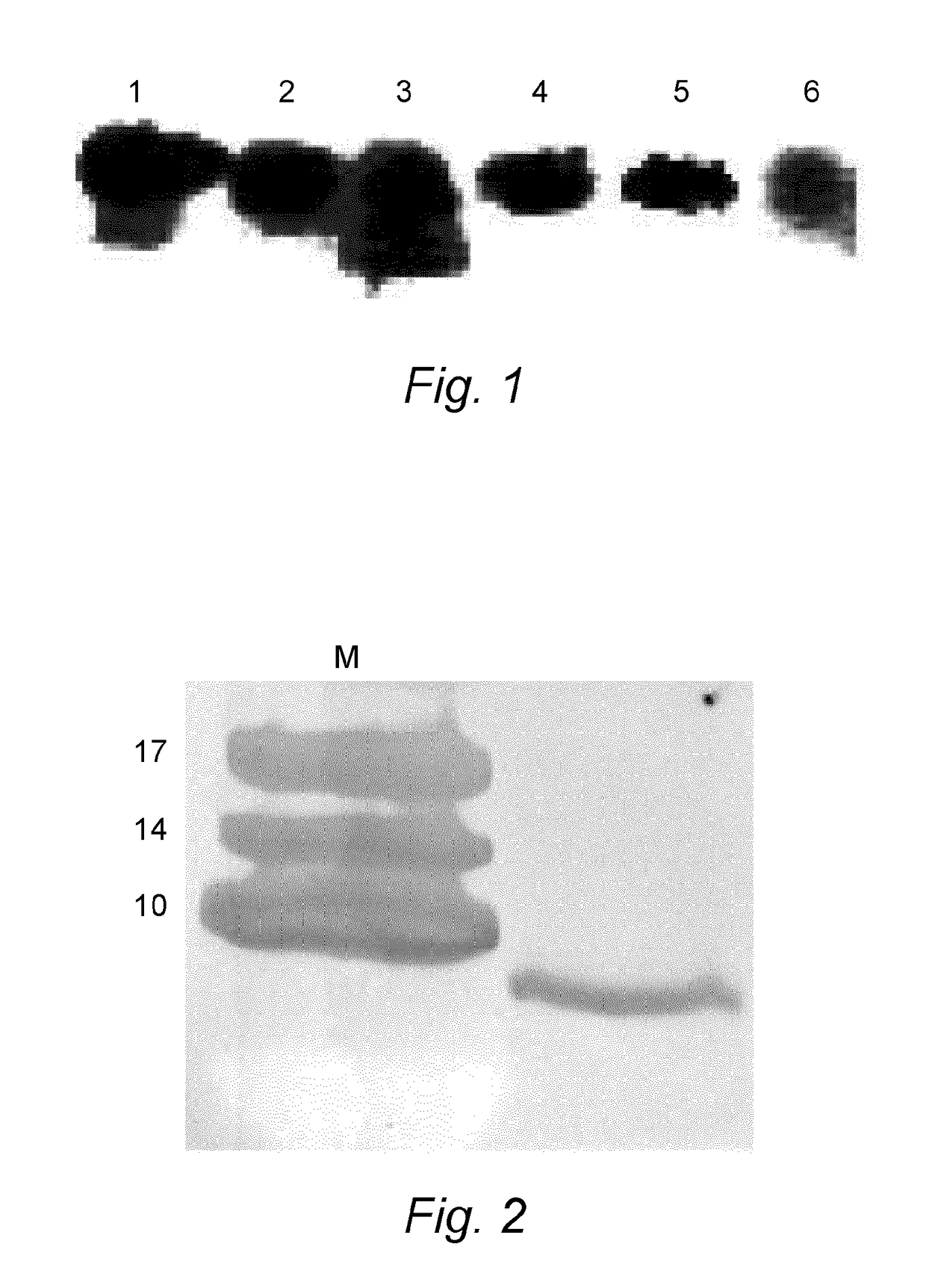
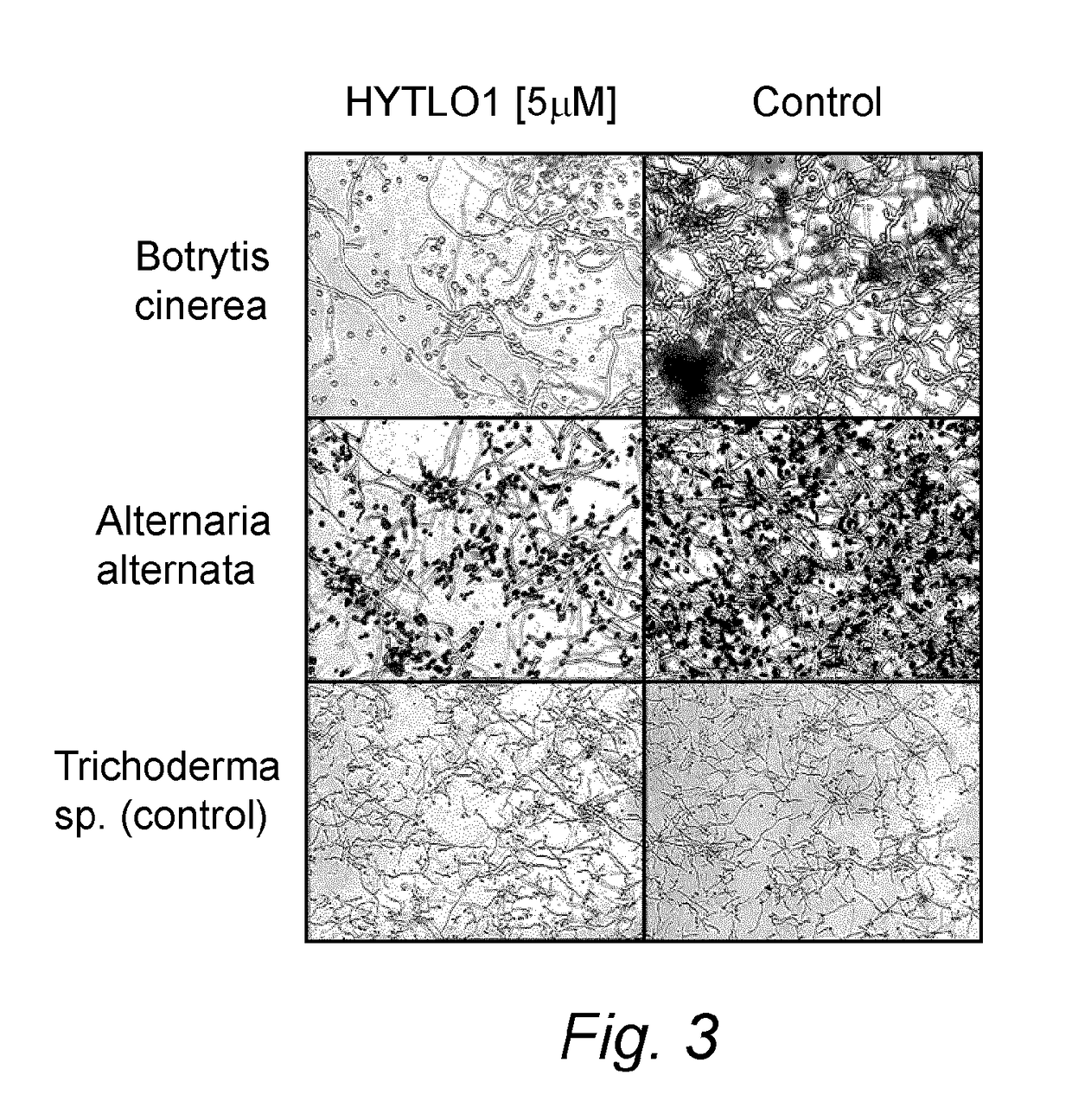
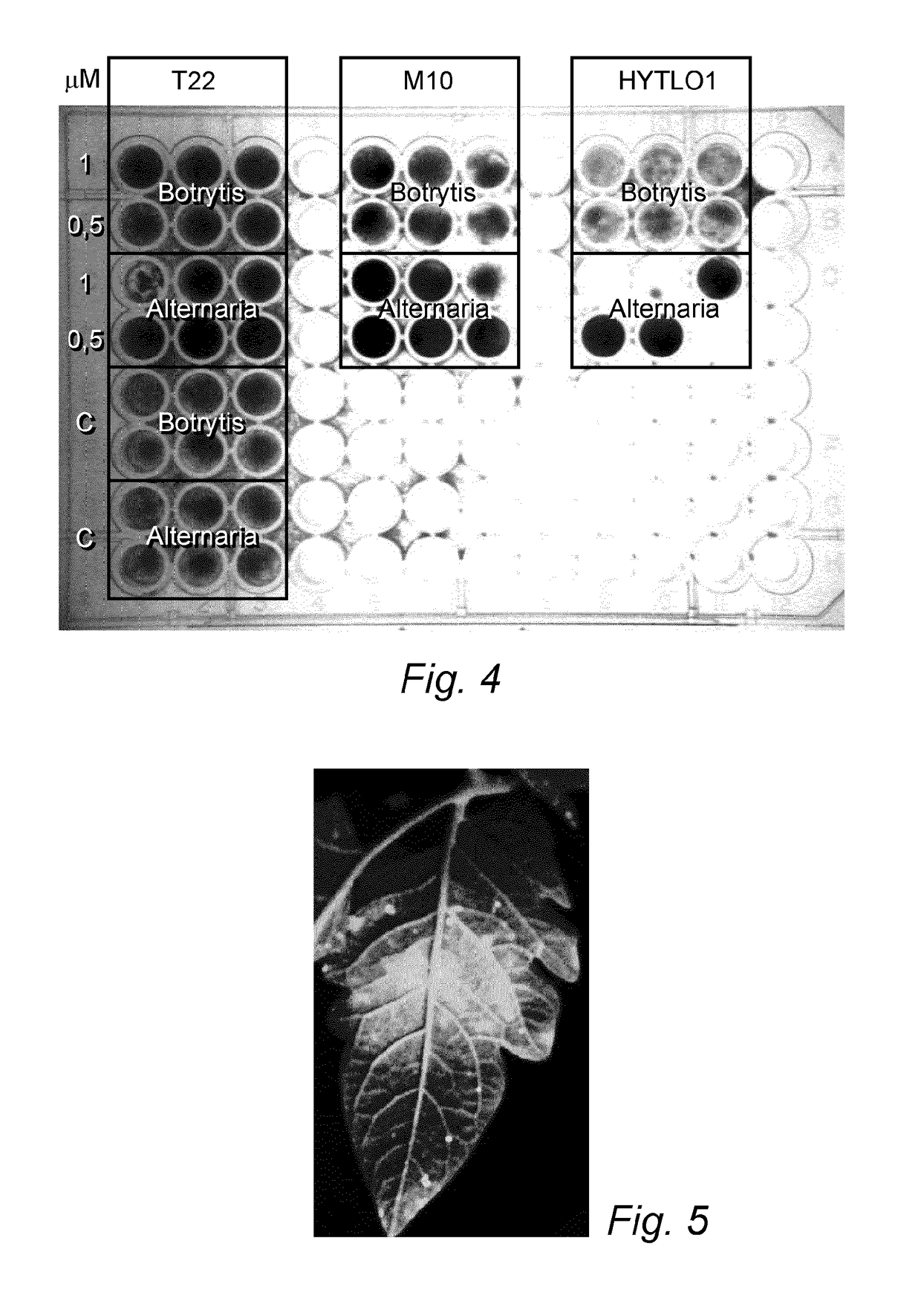
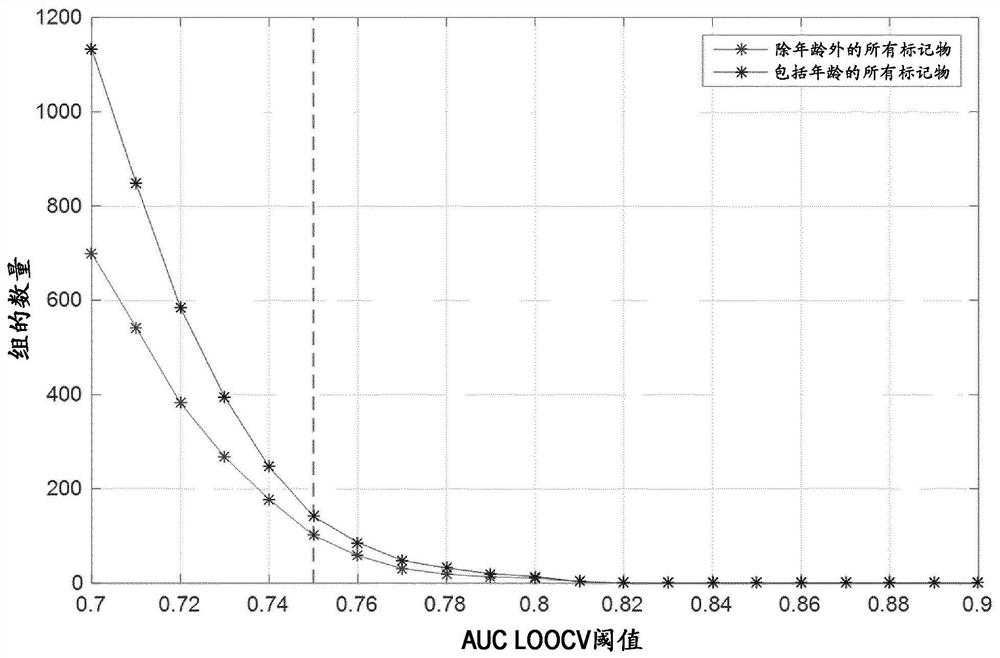
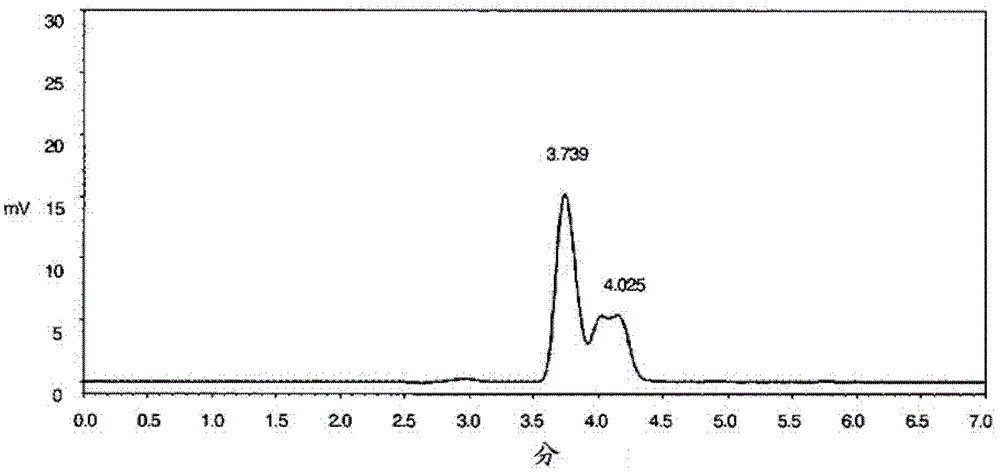
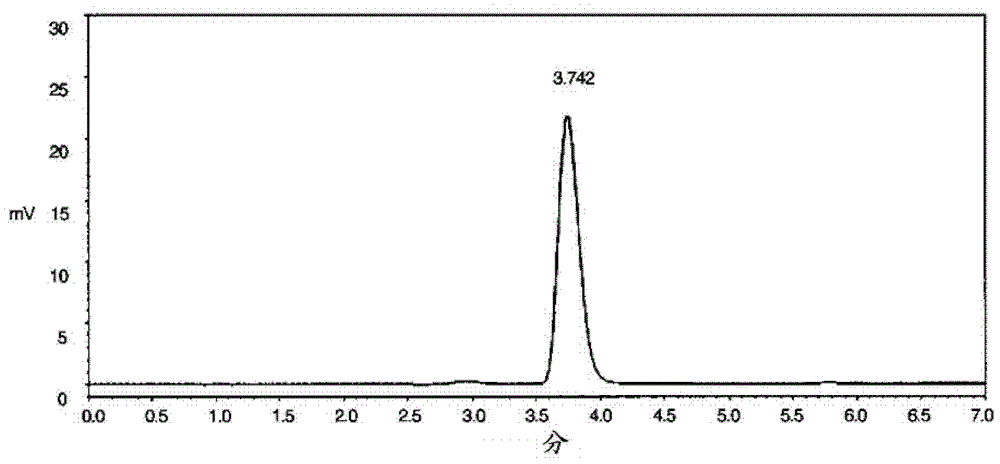
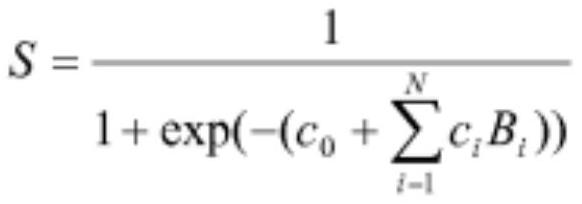Patents
Literature
66 results about "ESA Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
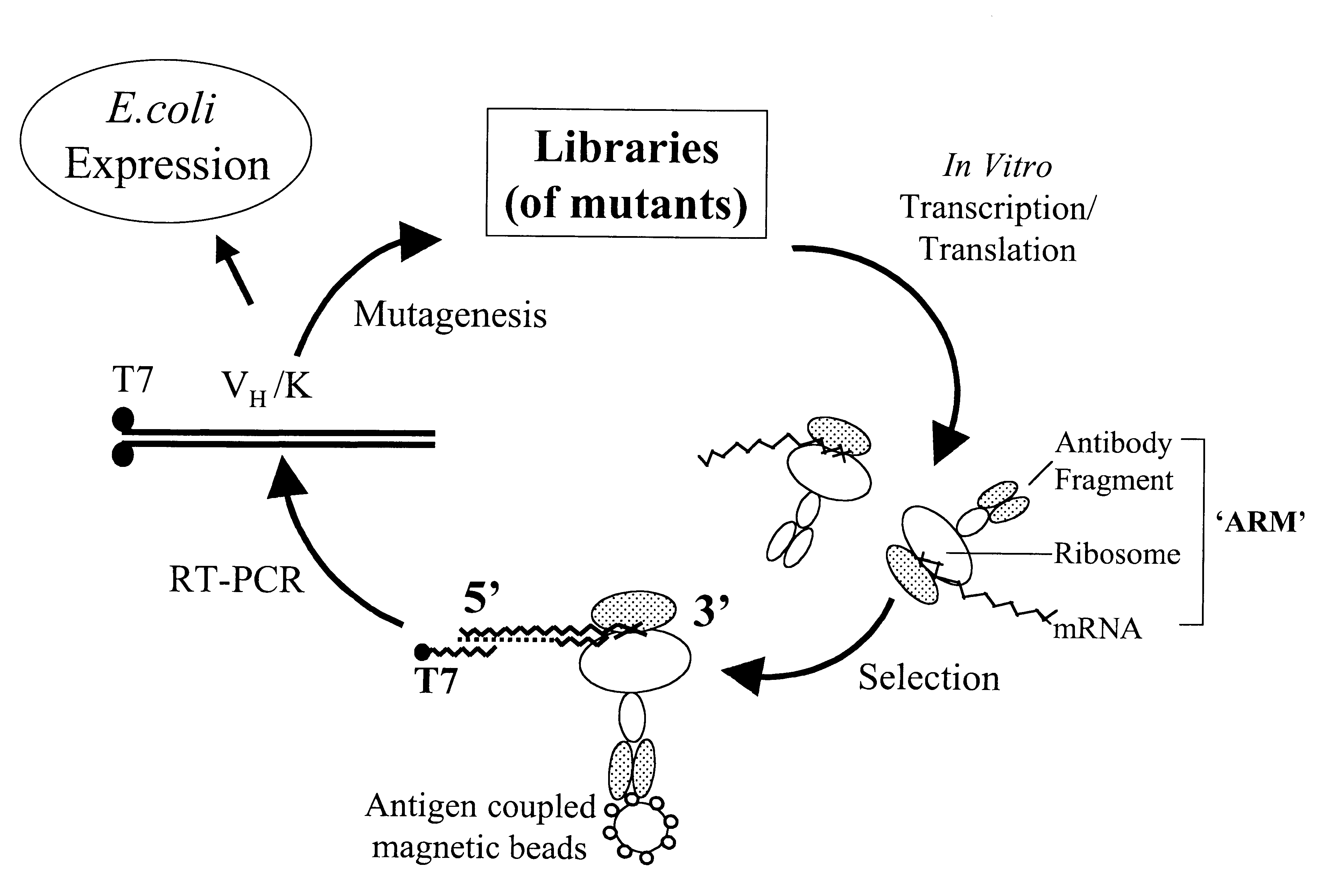
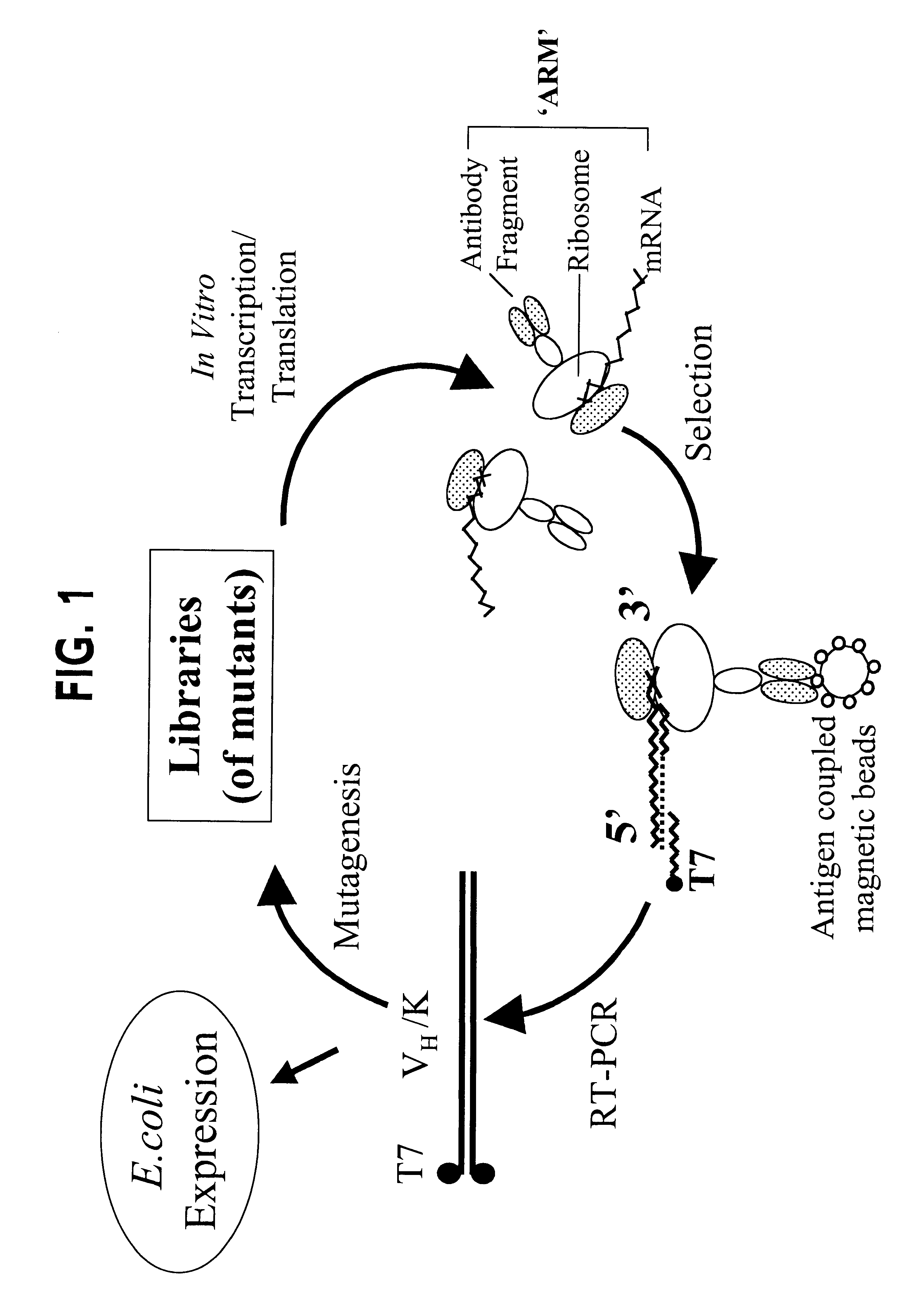
Ribosome complexes as selection particles for in vitro display and evolution of proteins
The invention provides a method of displaying nascent proteins or peptides as complexes with eukaryotic ribosomes and the mRNA encoding the protein or peptide following transcription and translation in vitro, of further selecting complexes carrying a particular nascent protein or peptide by means of binding to a ligand, antigen or antibody, and of subsequently recovering the genetic information encoding the protein or peptide from the selected ribosome complex by reverse transcription and polymerase chain reaction (RT-PCR). The RT-PCR recovery step is carried out directly on the intact ribosome complex, without prior dissociation to release the mRNA, thus contributing to maximal efficiency and sensitivity. The steps of display, selection and recovery can be repeated in consecutive cycles. The method is exemplified using single-chain antibody constructs as antibody-ribosome-mRNA complexes (ARMs).
Owner:CRESCENDO BIOLOGICS
IgA nephropathy-related DNA
InactiveUS6962984B2Reduce the burden onSugar derivativesMicrobiological testing/measurementWhite blood cellDna encoding
A novel DNA whose expression level fluctuates in leukocytes of IgA nephropathy patients in comparison with leukocytes of healthy persons, a process for isolating the DNA, a novel protein encoded by the DNA, an antibody recognizing the protein, methods for detecting the protein and the DNA, and methods of diagnosis and treatment of IgA nephropathy.
Owner:NIHON UNIVERSITY
Methods and compositions for treating solid tumors and enhancing tumor vaccines
The present invention provides methods of treating and enhancing efficacy of immunotherapy for a solid tumor in a subject, comprising the step of contacting the subject with a compound or composition that modulates the expression or activity of ETRB, ET-1, ICAM-1, or another protein found herein to play a role in homing of T cells to a solid tumor. The present invention also provides methods of prognosticating a solid tumor in a subject, comprising the step of measuring an expression level of a protein found herein to play a role in homing of T cells to a solid tumor, or a nucleotide molecule encoding same.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Molecules and chimeric molecules thereof
InactiveUS20090311247A1Peptide/protein ingredientsAntibody mimetics/scaffoldsProtein formationFlt3 ligand
The present invention relates generally to the fields of proteins, diagnostics, therapeutics and nutrition. More particularly, the present invention provides an isolated protein molecule such as EPO, Flt3-Ligand, Flt3, PDGF-B or VEGF-165 or chimeric molecules thereof comprising at least a portion of the protein molecule, wherein the protein or chimeric molecule has a profile of measurable physiochemical parameters which is indicative of, associated with or forms the basis of, one or more pharmacological traits. The present invention further contemplates the use of the isolated protein or chimeric molecule thereof in a range of diagnostic, prophylactic, therapeutic, nutritional and / or research applications.
Owner:APOLLO LIFE SCI
Antisense oligonucleotide directed removal of proteolytic cleavage sites from proteins
ActiveUS20130198877A1Improve the level ofLow toxicitySplicing alterationNervous disorderPrecursor mRNAProteolysis
Described are means and methods for removing a proteolytic cleavage site from a protein, the method comprising providing a cell that expresses pre-mRNA encoding the protein with an anti-sense oligonucleotide that induces skipping of the exonic sequence that encodes the proteolytic cleavage site, and allowing translation of mRNA produced from the pre-mRNA.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
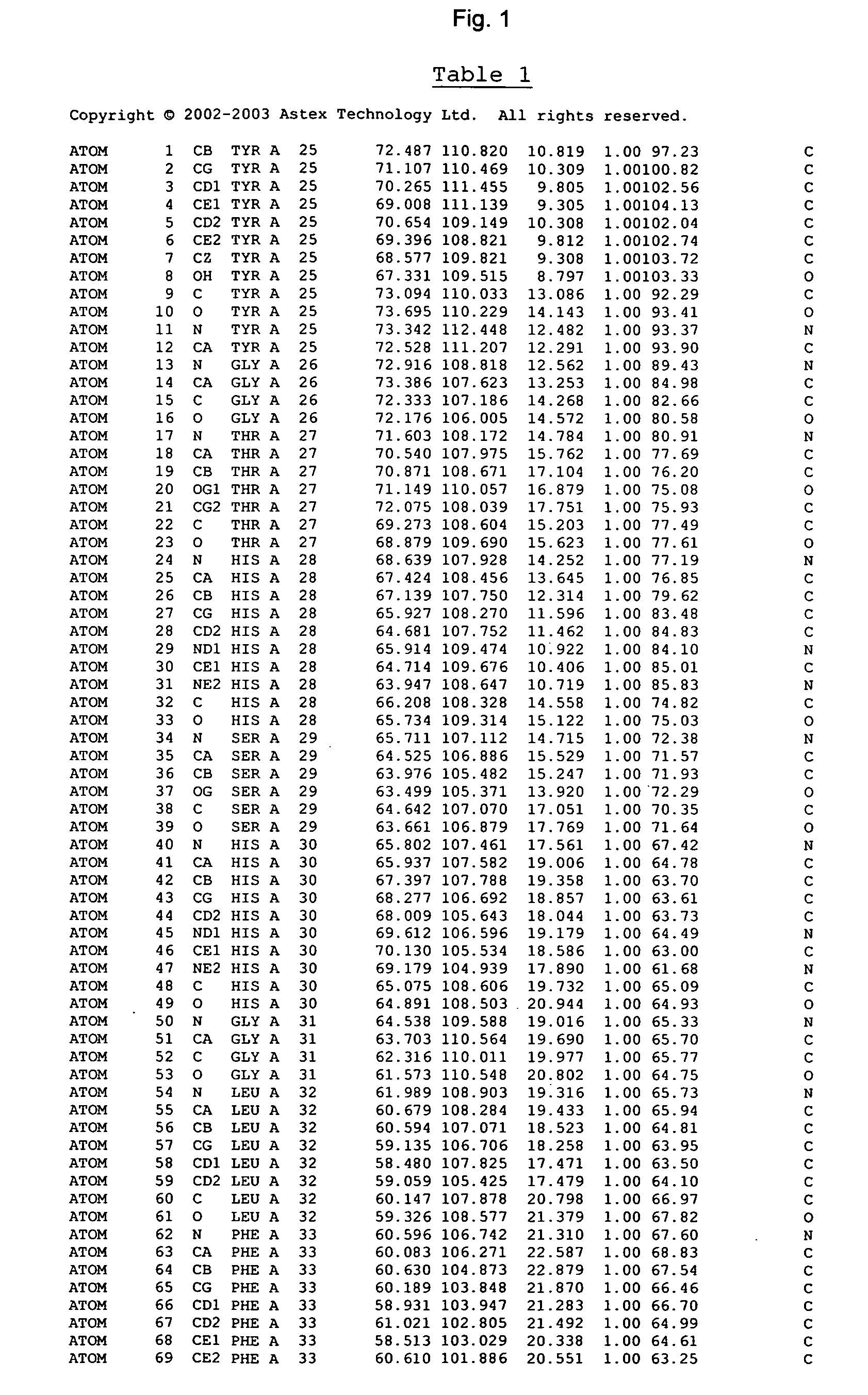
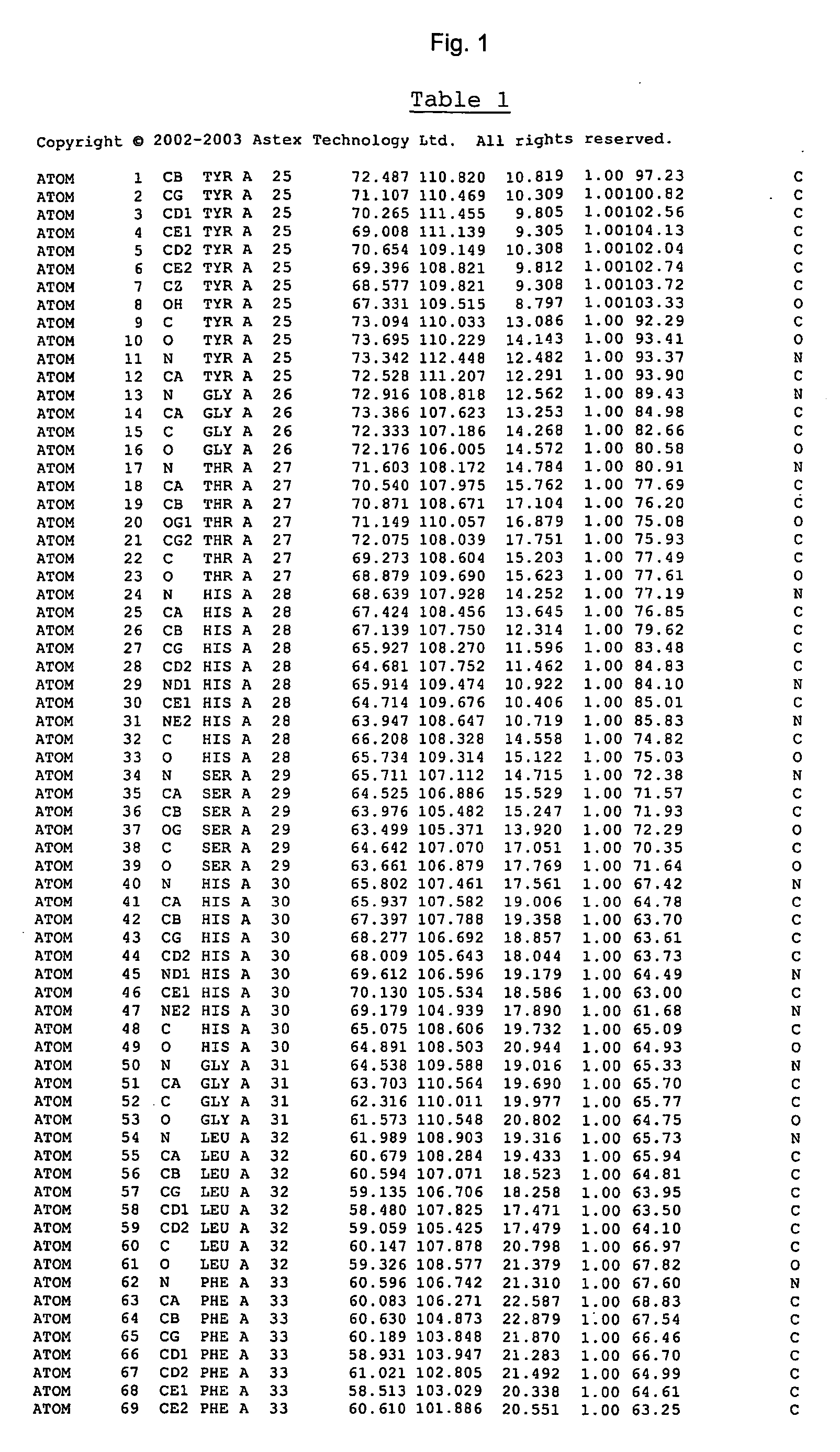
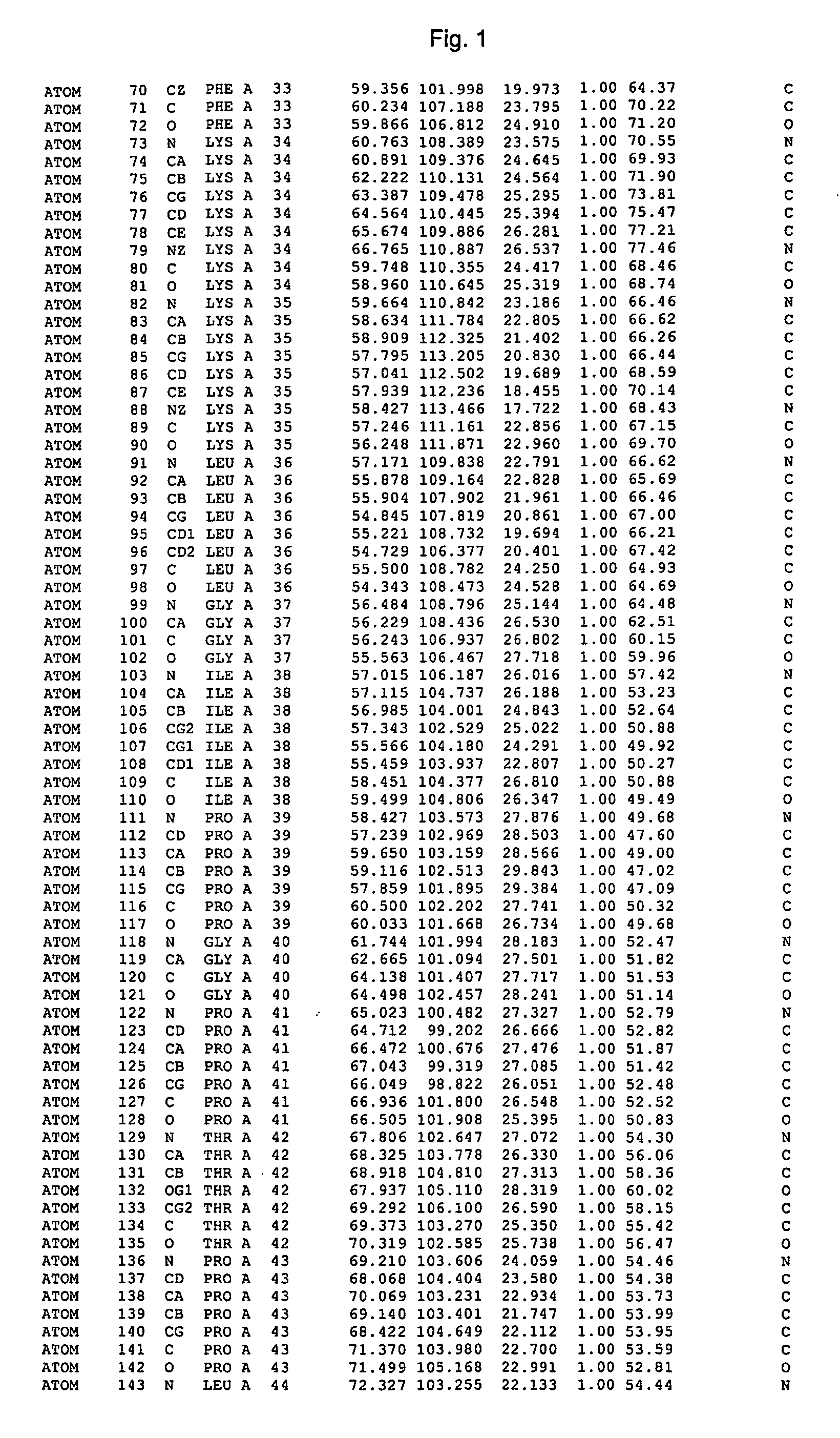
Crystal structure of cytochrome P450
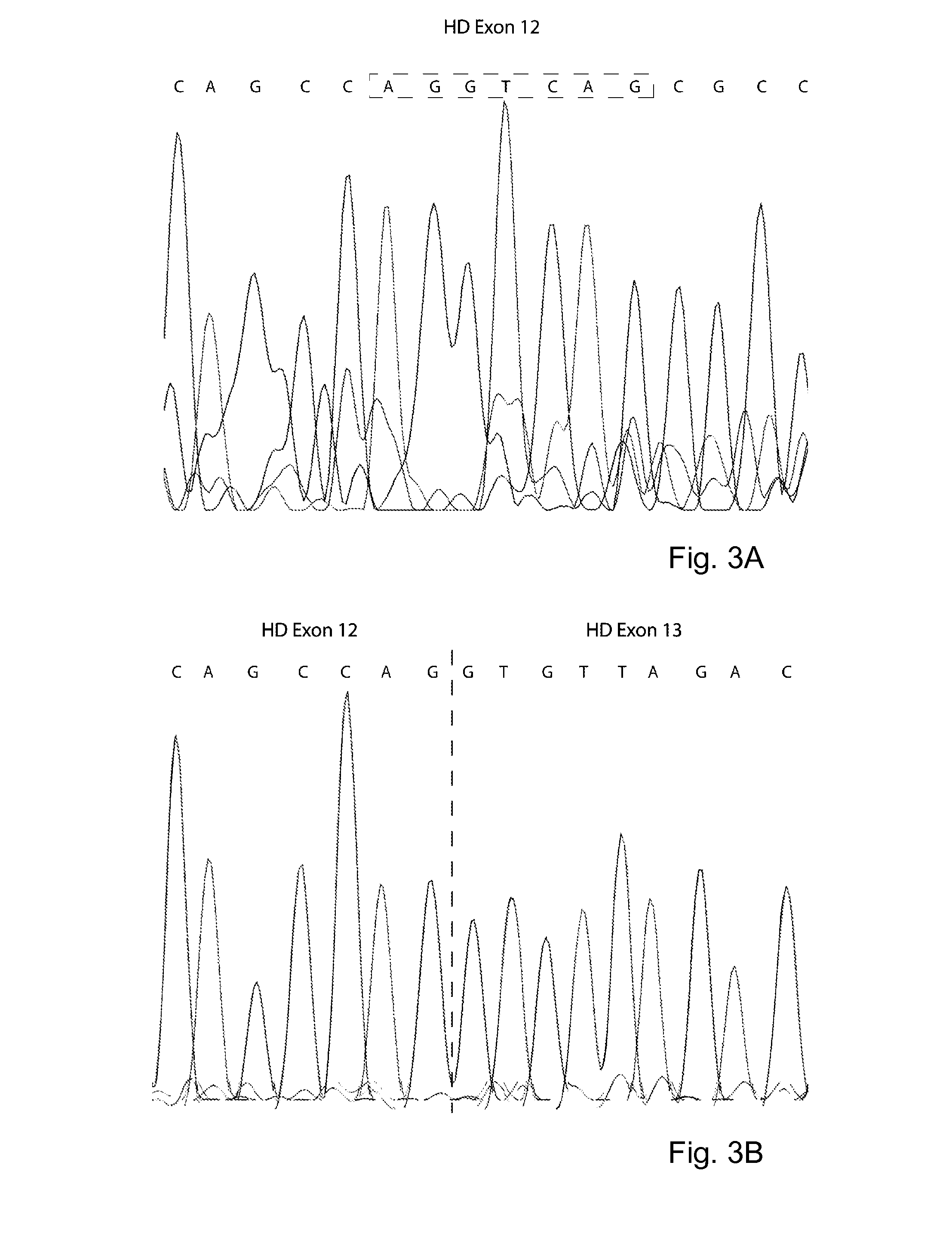
The invention provides the crystal structure of the cytochrome P450 3A4 protein molecule. The structure is set out in Table 5. The structure may be used in to model the interaction of compounds such as pharmaceuticals with this protein, and to determine the structure of related cytochrome P450 molecules.
Owner:ASTEX THERAPEUTICS LTD
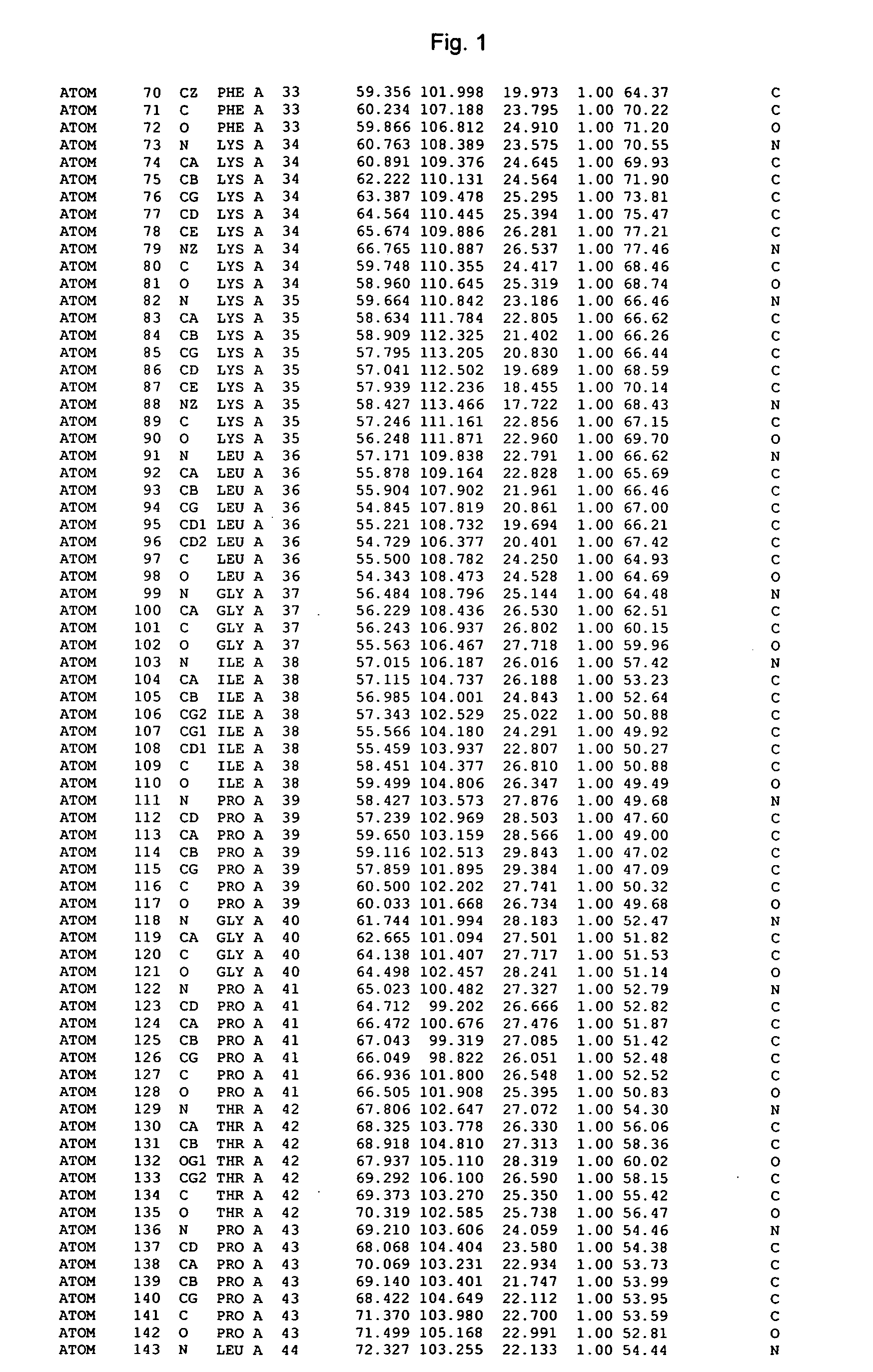
Crystal structure of cytochrome P450
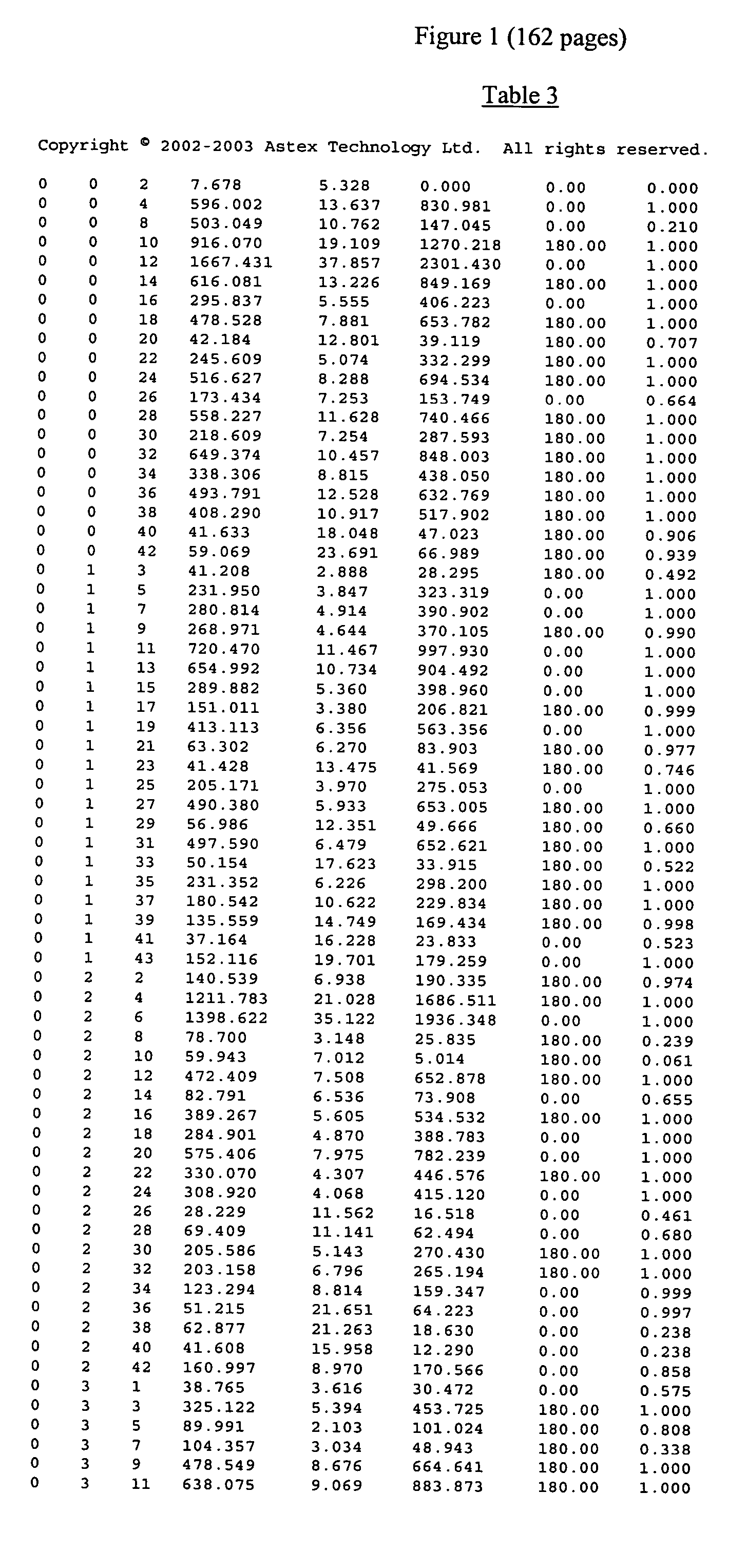
The invention provides the crystal structure of the cytochrome P450 3A4 protein molecule. The structure is set out in Tables 1-4. The structure may be used in to model the interaction of compounds such as pharmaceuticals with this protein, and to determine the structure of related cytochrome P450 molecules.
Owner:ASTEX THERAPEUTICS LTD
Protein designated PEG modification method and obtained PEG modified protein
InactiveCN104130308AExtended half-lifeEasy to operatePeptide/protein ingredientsAntiviralsESA ProteinBio engineering
The invention belongs to the technical field of biological engineering, and discloses a protein designated PEG modification method and an obtained PEG modified protein. The protein designated PEG modification method of the invention is as below: acquiring a protein, fusing a sorting motif recognized by a sortase at the C terminal of the protein to obtain a recombinant protein; mixing a PEG modifier, the recombinant protein and sortase, conducting peptide bond cutting and connection reaction to obtain a PEG modified protein, wherein the PEG modifier has a structure shown in a formula (I). The invention utilizes the sortase to realize designated PEG modification at the C terminal of the protein; the whole operation is simple and low in cost, avoids multisite modification in protein PEG modification, is more conducive to application of PEG modification in protein modification; m represents the degree of polymerization, and satisfies the relation of 1<=m<= 5; and n represents the degree of polymerization, and satisfies the relation of 110<= n<=1100.
Owner:JILIN UNIV
Compositions and methods for detecting cancers in a subject
InactiveUS20100047830A1Immunoglobulins against animals/humansBiological material analysisAntigenESA Protein
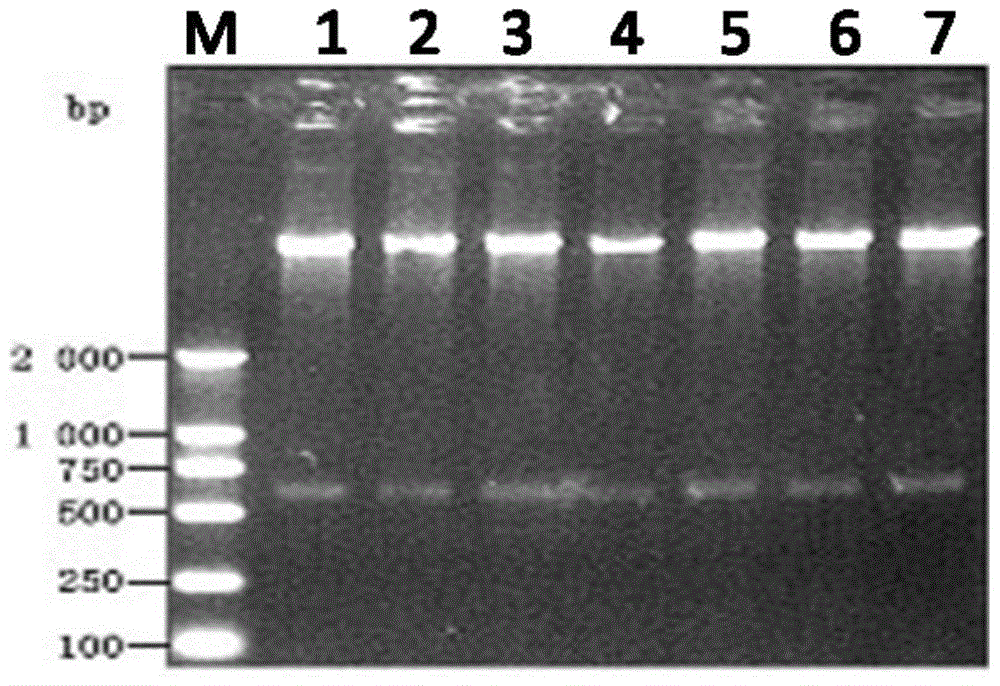
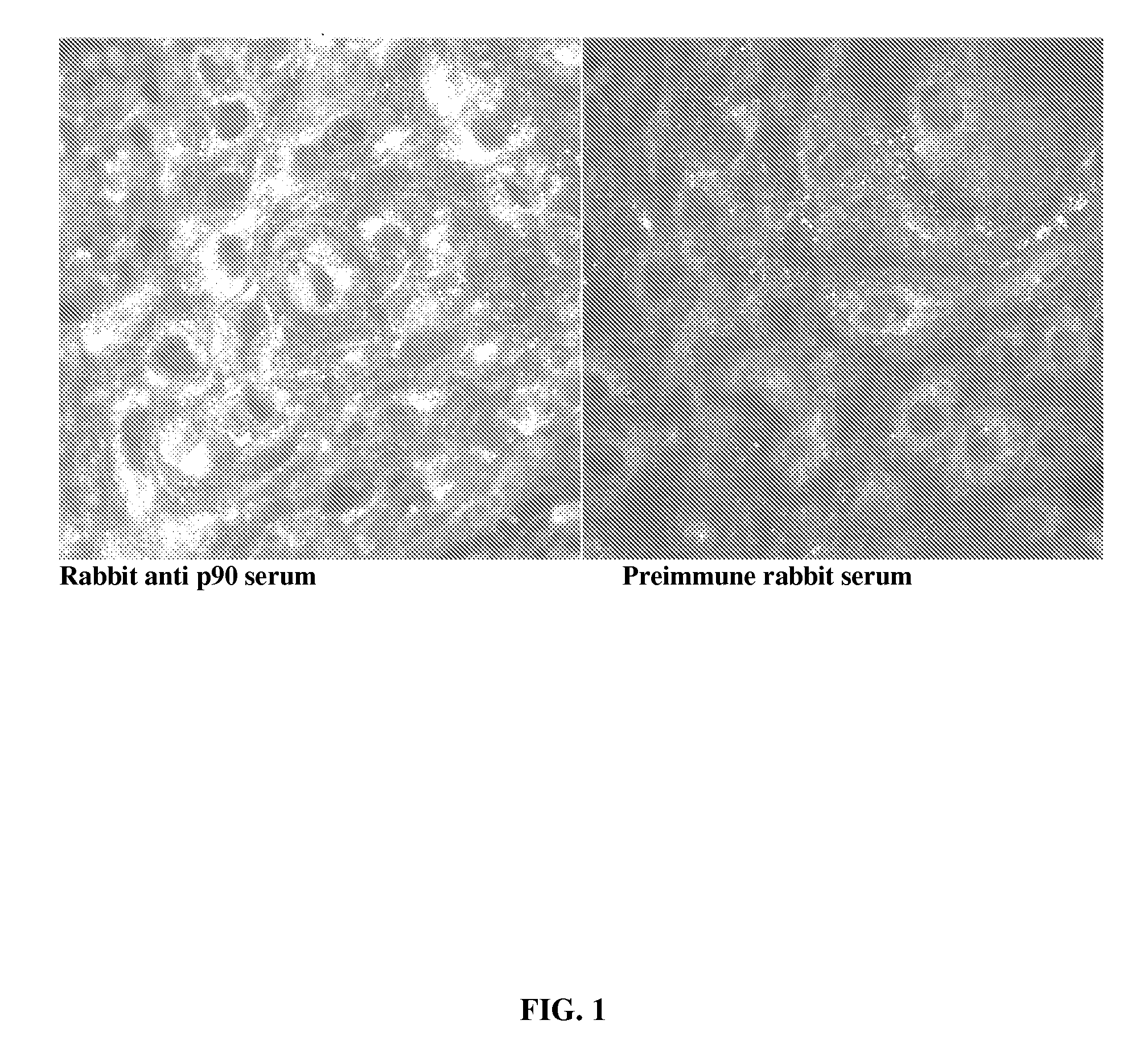
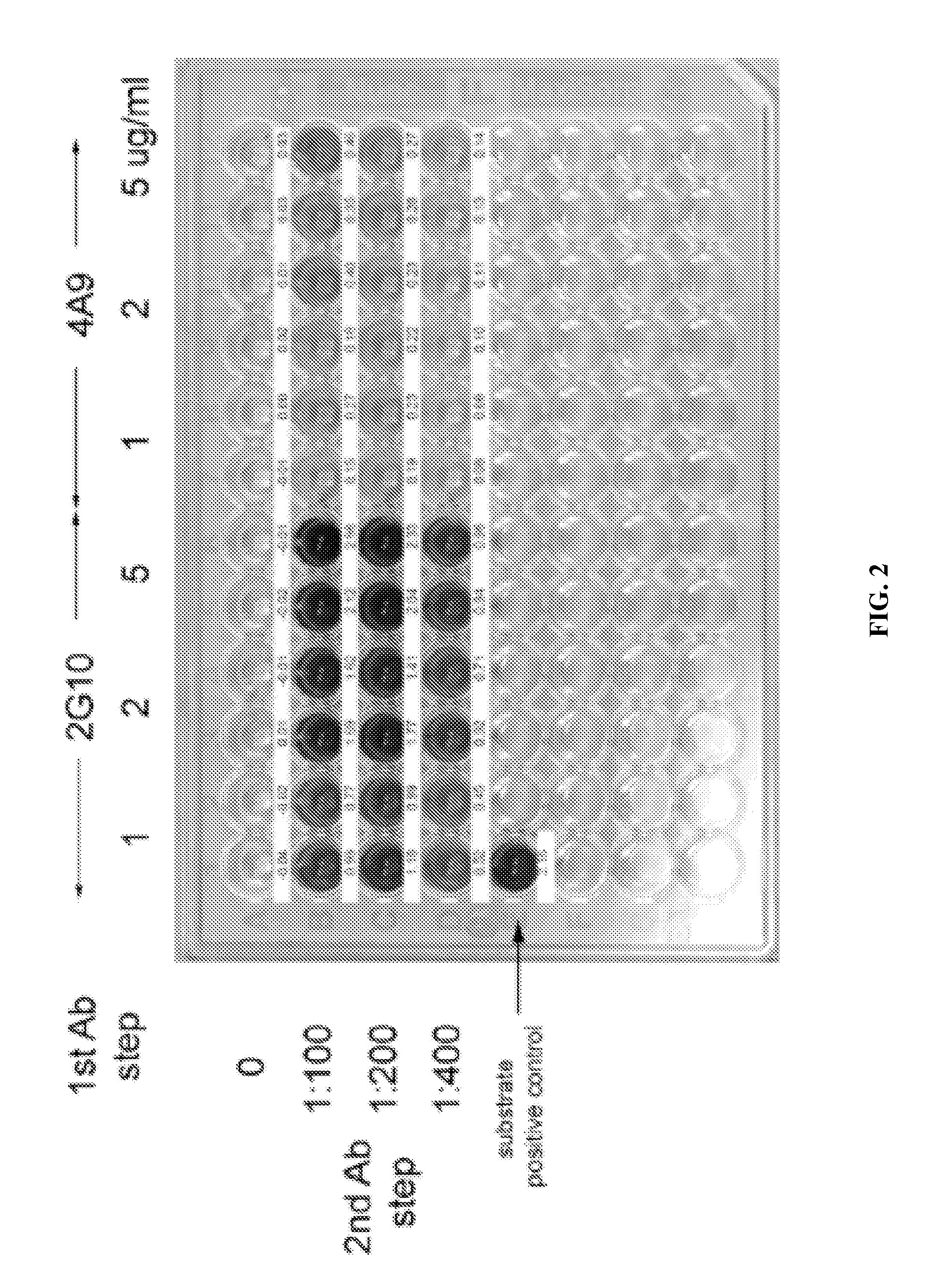
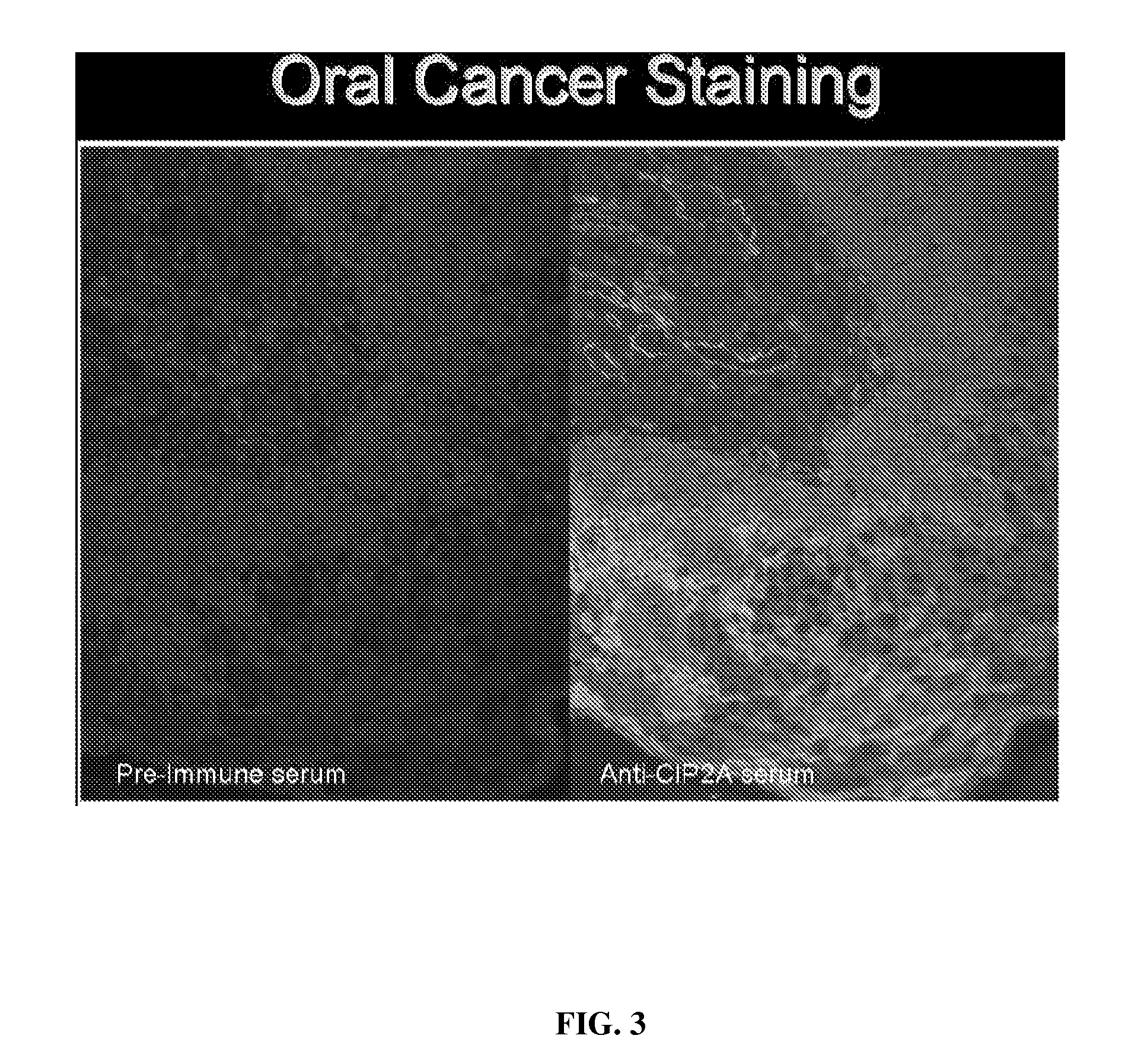
Disclosed are compositions and methods for detecting oral and gastrointestinal cancers in a subject. Autoantigen p90 was shown to be overexpressed in oral cancer cells, and this protein, as well as its companion autoantigen p62 and antibodies directed to both proteins, can be used as markers for detecting oral digestive and other cancers in a subject at an early stage.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
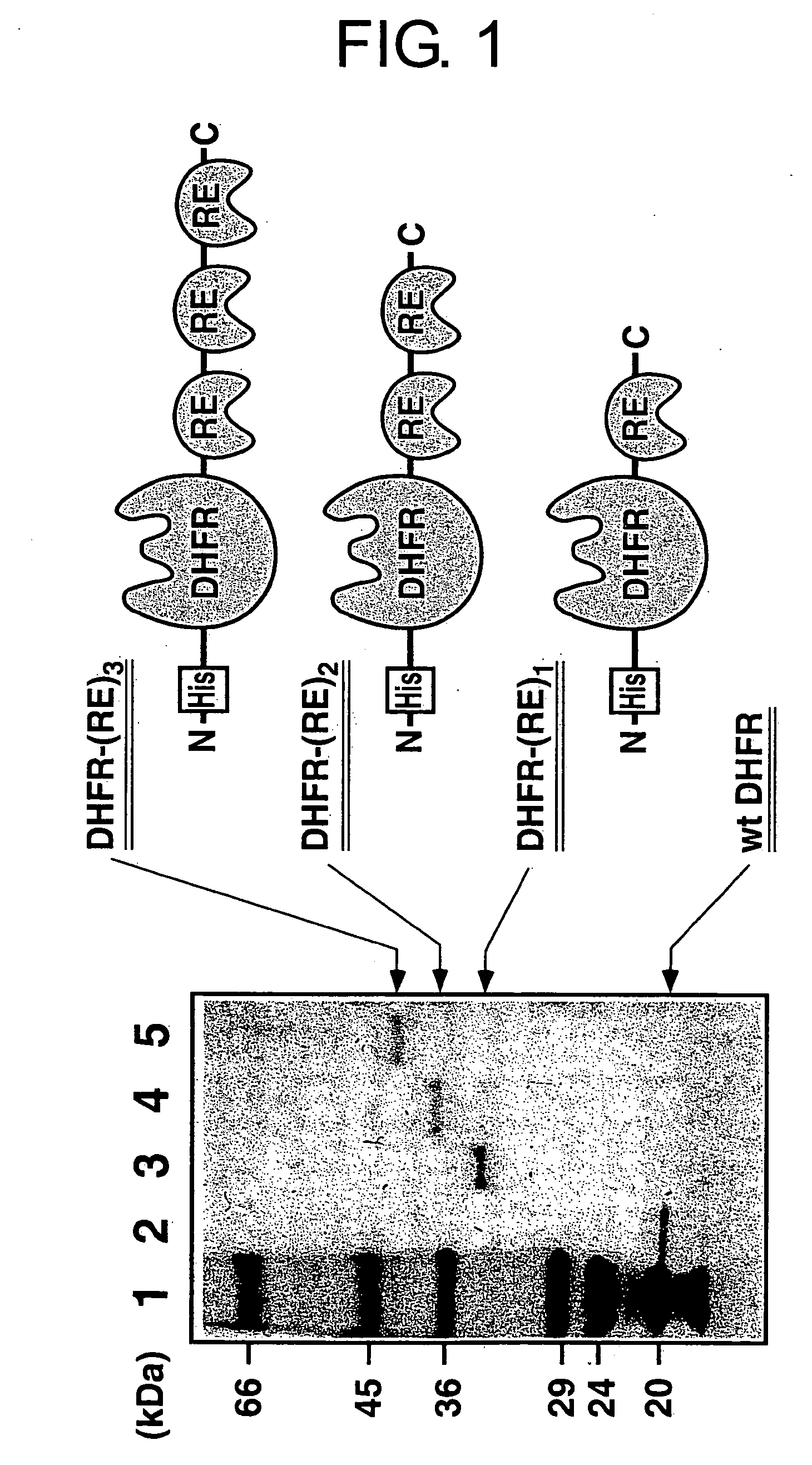
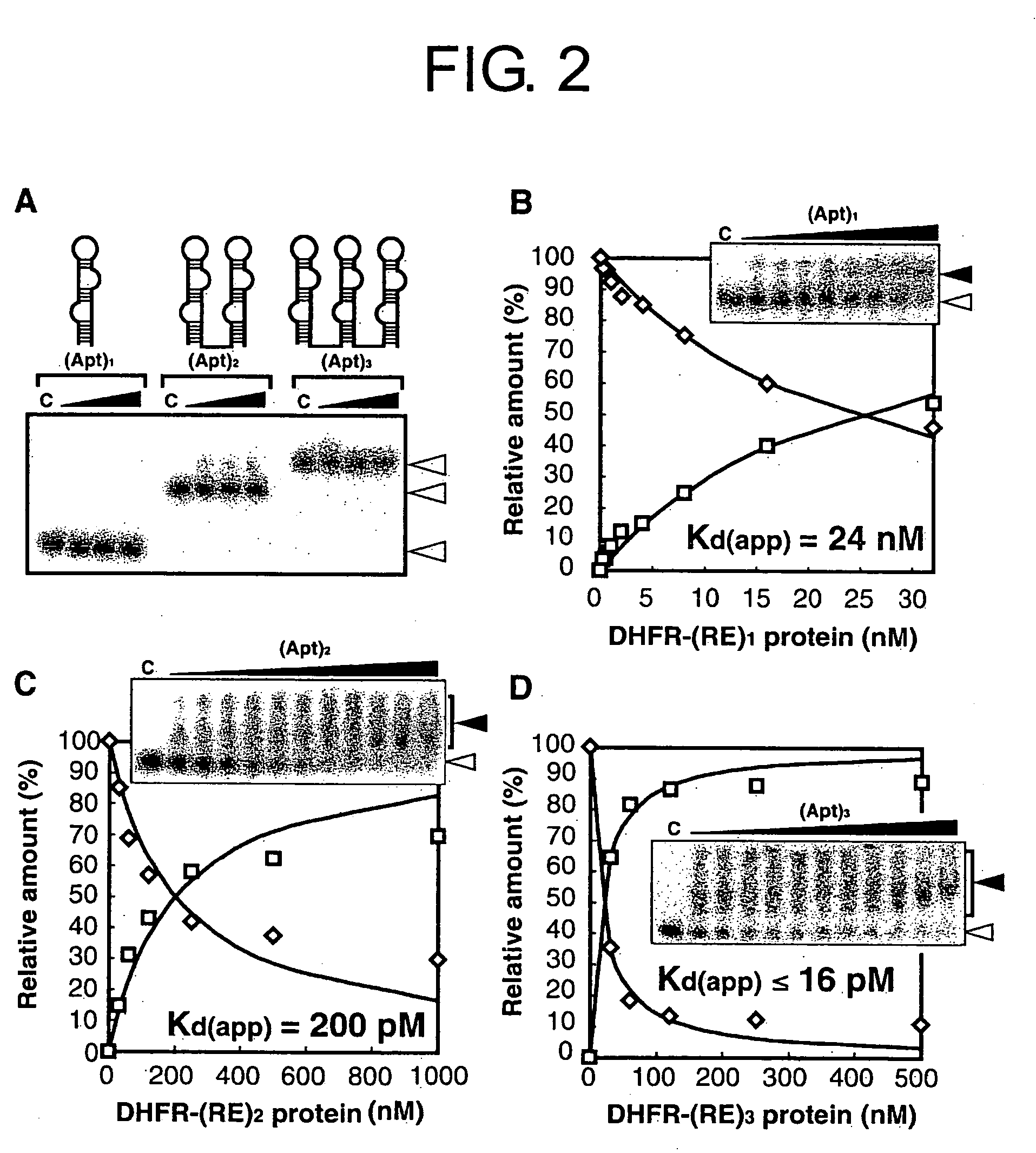
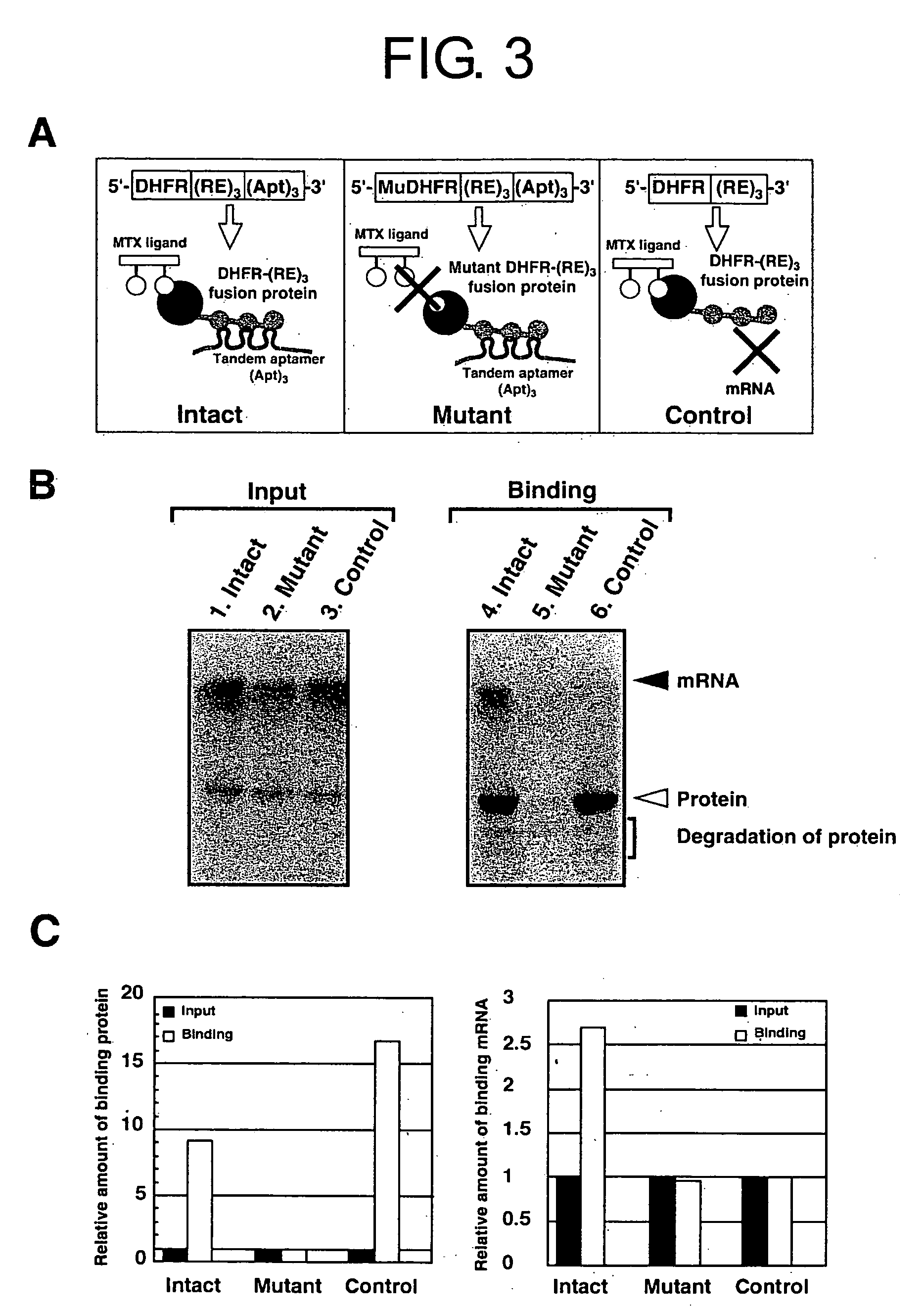
Method for forming a stable complex comprising a transcription product and translation product of a dna encoding a desired polypeptide, a nucleic acid construct used for the method, a complex formed by the method, and screening of a functional protein and mrna or dna encoding the protein using the method
InactiveUS20050191626A1Without reducing sequence varietyLinkage stabilitySugar derivativesMicrobiological testing/measurementGenotypeA-DNA
A stable linkage between a genotype and a phenotype in a cell-free system was successfully achieved by using interaction between a RNA-binding protein and RNA, between a DNA-binding protein and DNA, or by using a protein that inactivates a ribosome. Furthermore, it was found that functional proteins could be selected by using these stable linkages.
Owner:NAT INST OF ADVANCED IND SCI & TECH
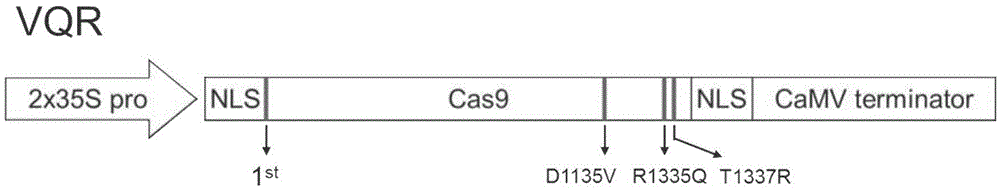
Plant Cas9 variant protein VQR as well as encoding gene and application thereof
The invention discloses plant Cas9 variant protein VQR. An amino acid sequence of the protein is shown as SEQ ID No: 2. The invention also discloses a plant Cas9 variant gene VQR. The gene encodes the plant Cas9 variant protein VQR, and a nucleotide sequence of the gene is shown as SEQ ID No: 1. The invention further discloses a recombinant expression vector containing the gene. The plant Cas9 variant protein VQR has the application of being capable of achieving a genome editing function under the condition that the PAM (protospacer adjacent motif) region is 5'-NGA-3'.
Owner:CHINA NAT RICE RES INST
Crystal structure of cytochrome P450 3A4 and uses thereof
InactiveUS20070179716A1Microbiological testing/measurementBiological material analysisESA ProteinProtein molecules
The invention provides the crystal structure of the cytochrome P450 3A4 protein molecule. The structure is set out in Tables 1-4. The structure may be used in to model the interaction of compounds such as pharmaceuticals with this protein, and to determine the structure of related cytochrome P450 molecules.
Owner:ASTEX THERAPEUTICS LTD
System and method for prediction of protein-ligand bioactivity and pose propriety
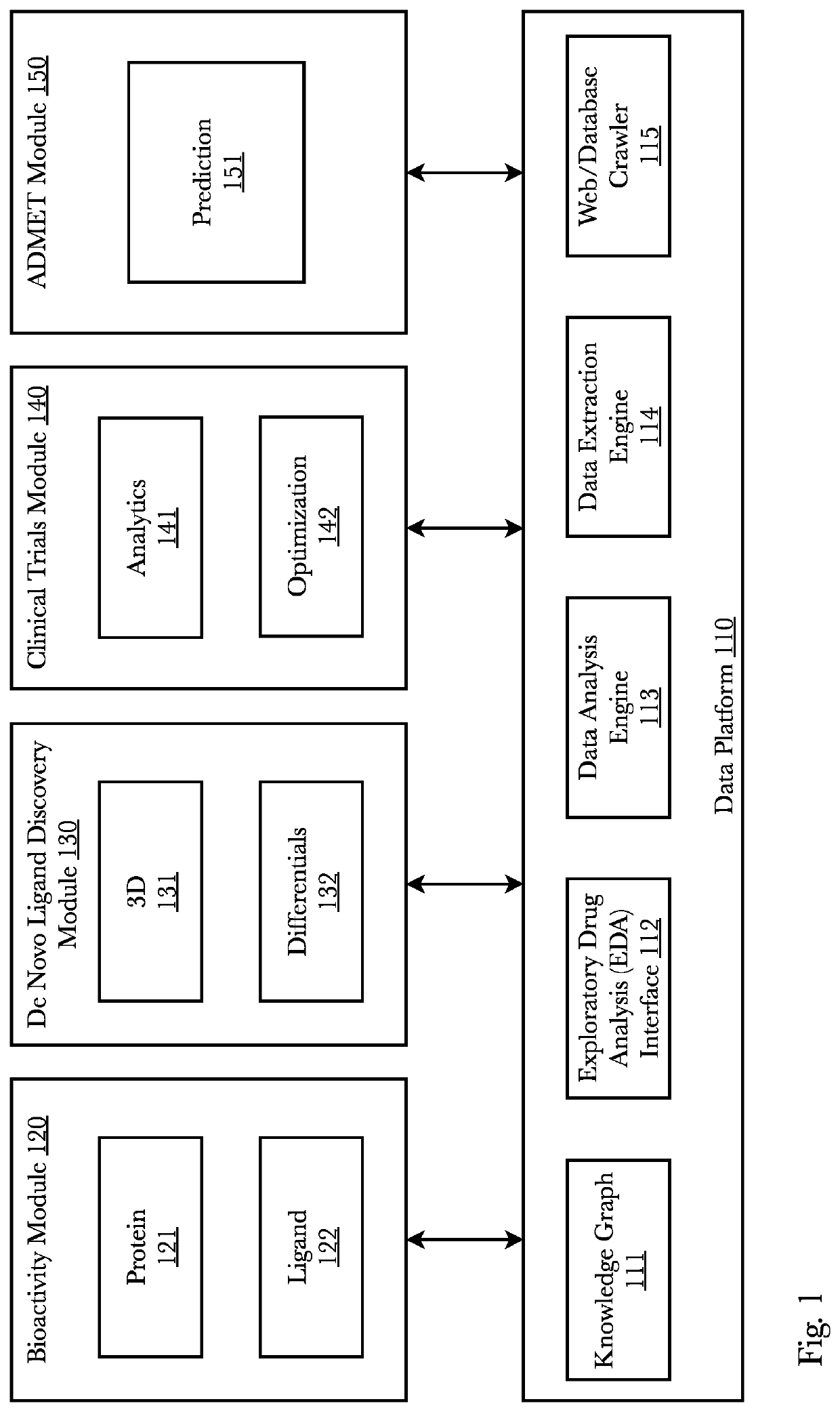
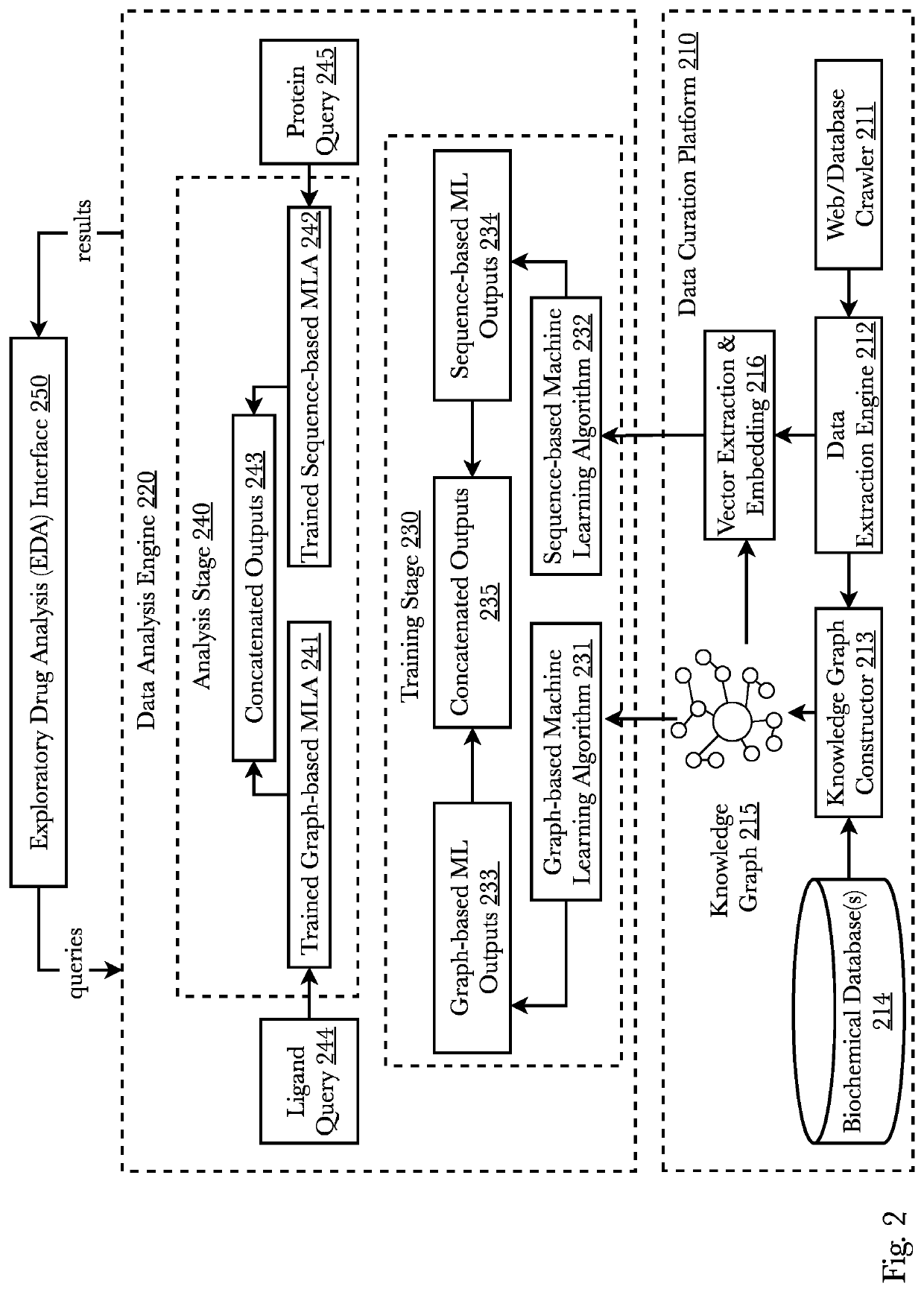
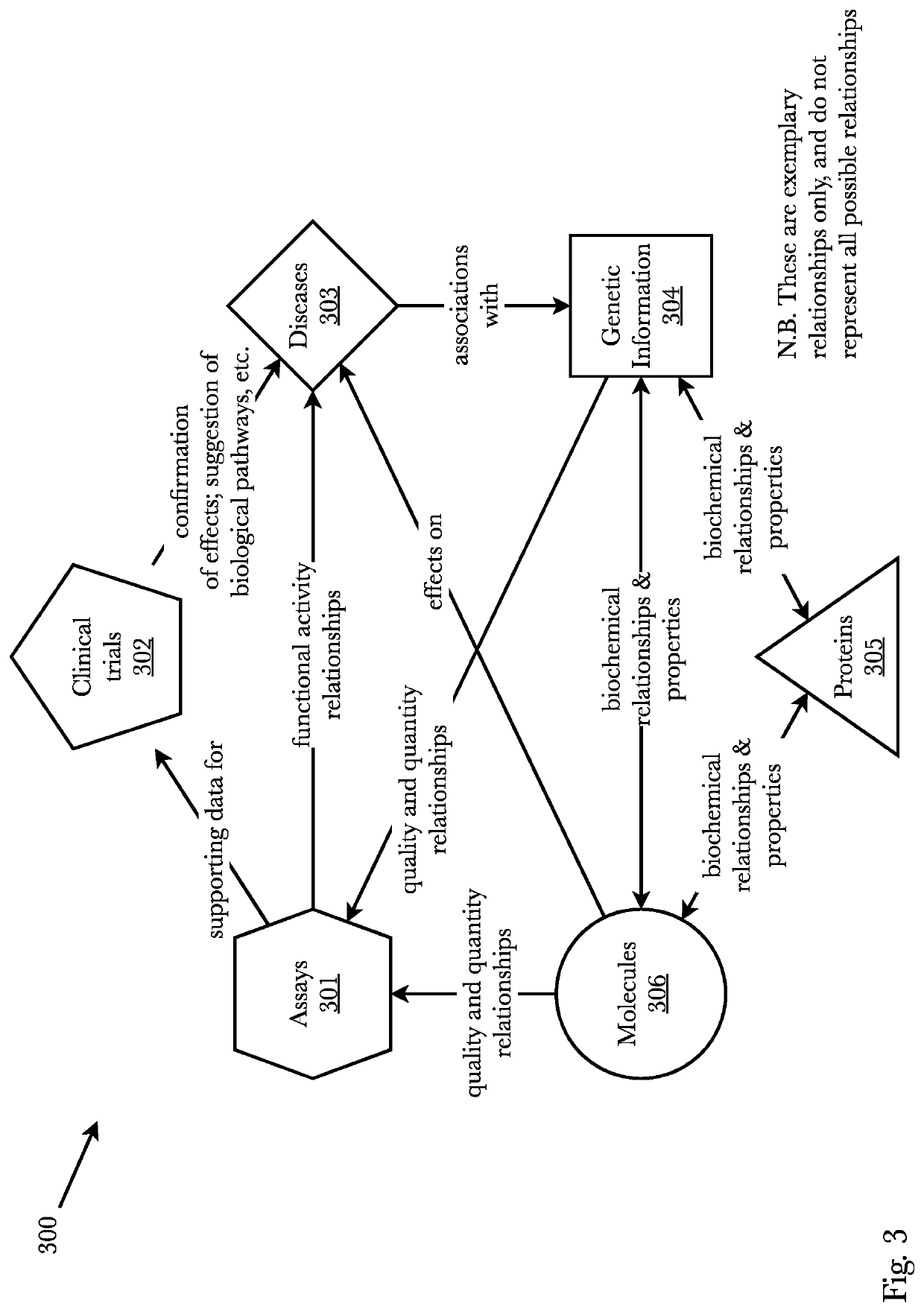
ActiveUS11256994B1Enhance machine learning modelEnhance the machine learning modelWeb data indexingAnalogue computers for chemical processesProtein targetExperimental laboratory
A system and method that predicts whether a given protein-ligand pair is active or inactive and outputs a pose score classifying the propriety of the pose. A 3D bioactivity platform comprising a 3D bioactivity module and data platform scrapes empirical lab-based data that a docking simulator uses to generate a dataset from which a 3D-CNN model is trained. The model then may receive new protein-ligand pairs and determine a classification for the bioactivity and pose propriety of that protein-ligand pair. Furthermore, gradients relating to the binding affinity in the 3D model of the molecule may be used to generate profiles from which new protein targets may be determined.
Owner:RO5 INC
150 Kda accessory receptor for tgf-beta
InactiveUS20040191860A1Good effectPeptide/protein ingredientsMammal material medical ingredientsADAMTS ProteinsMutant
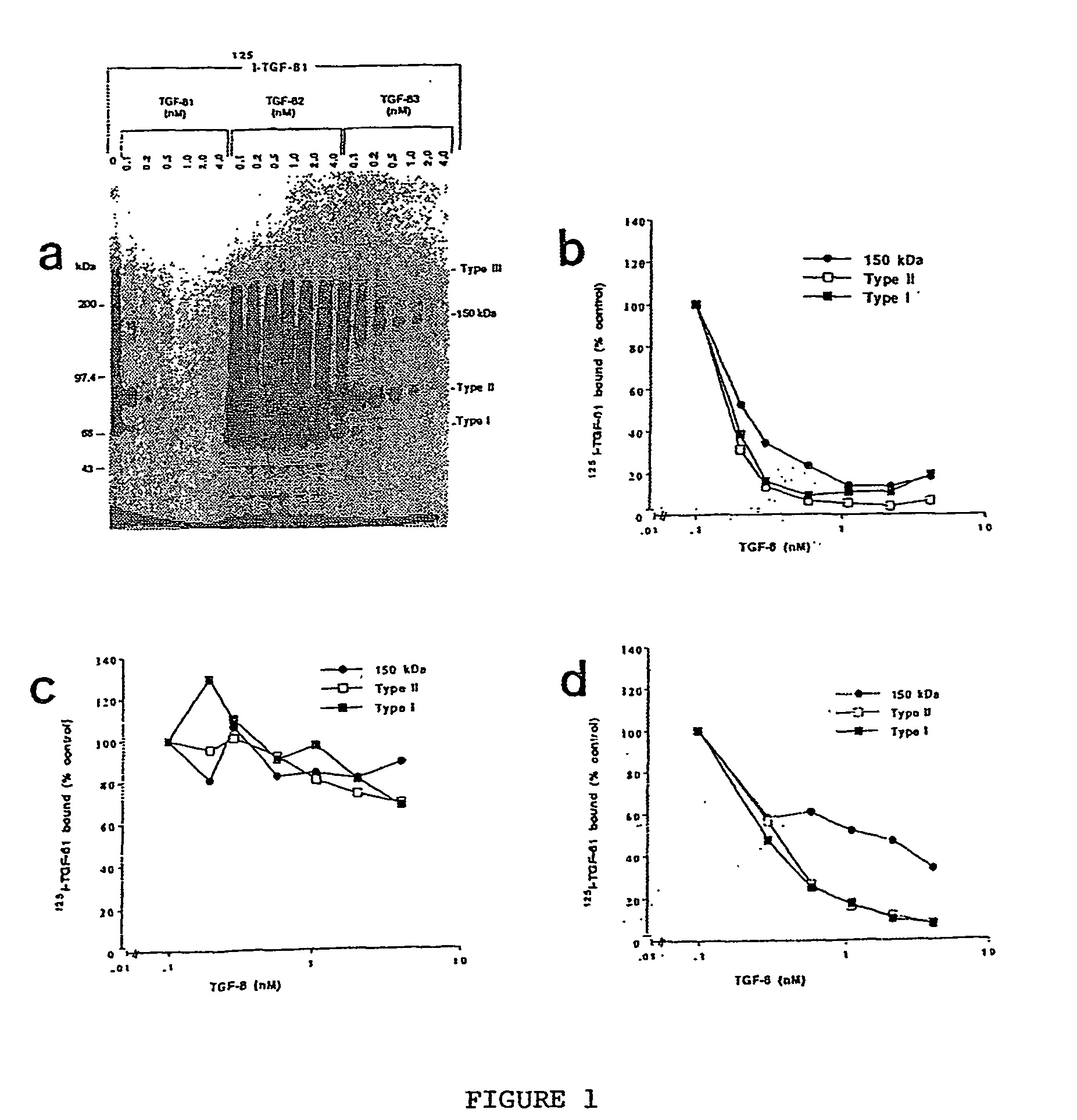
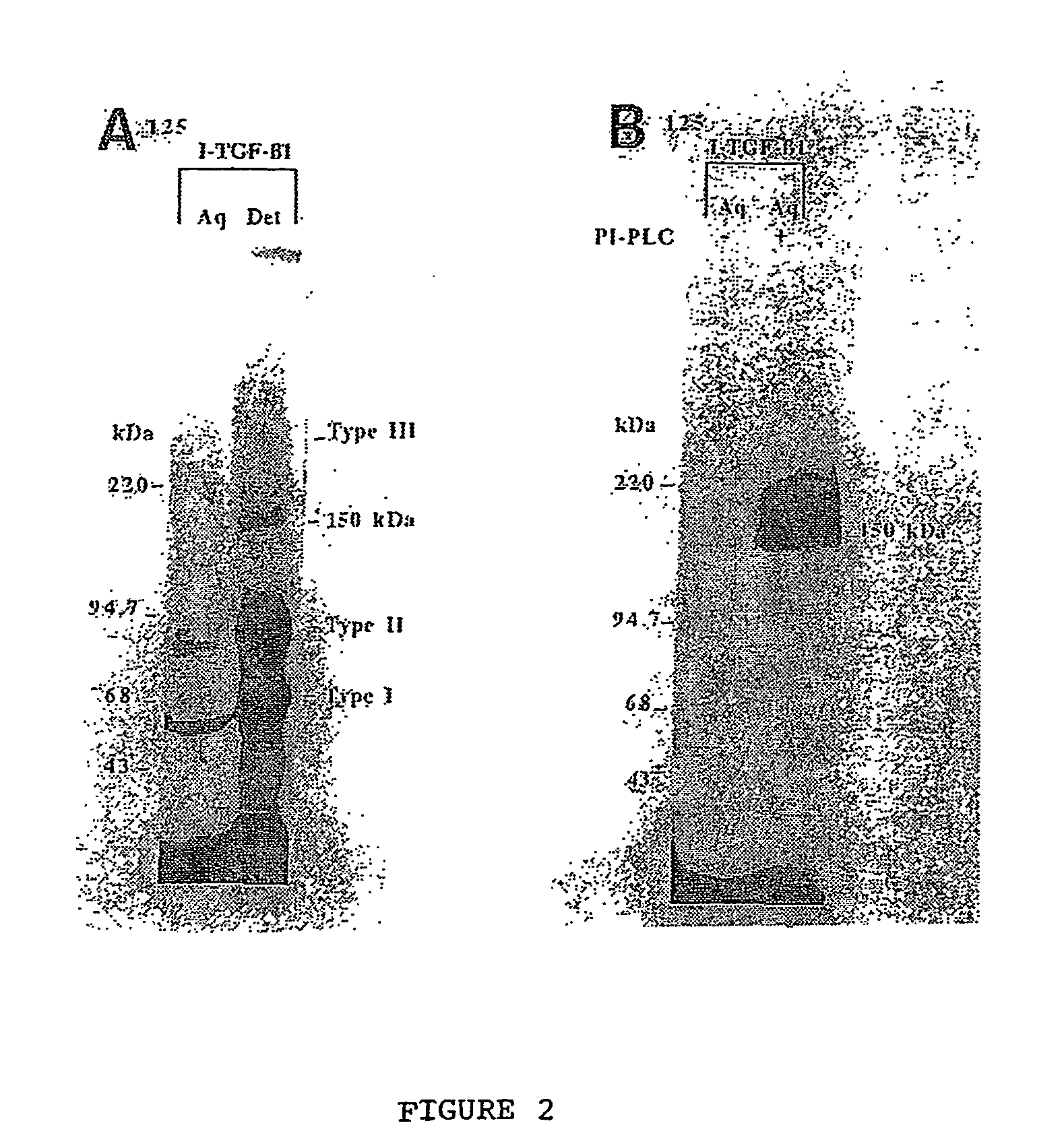
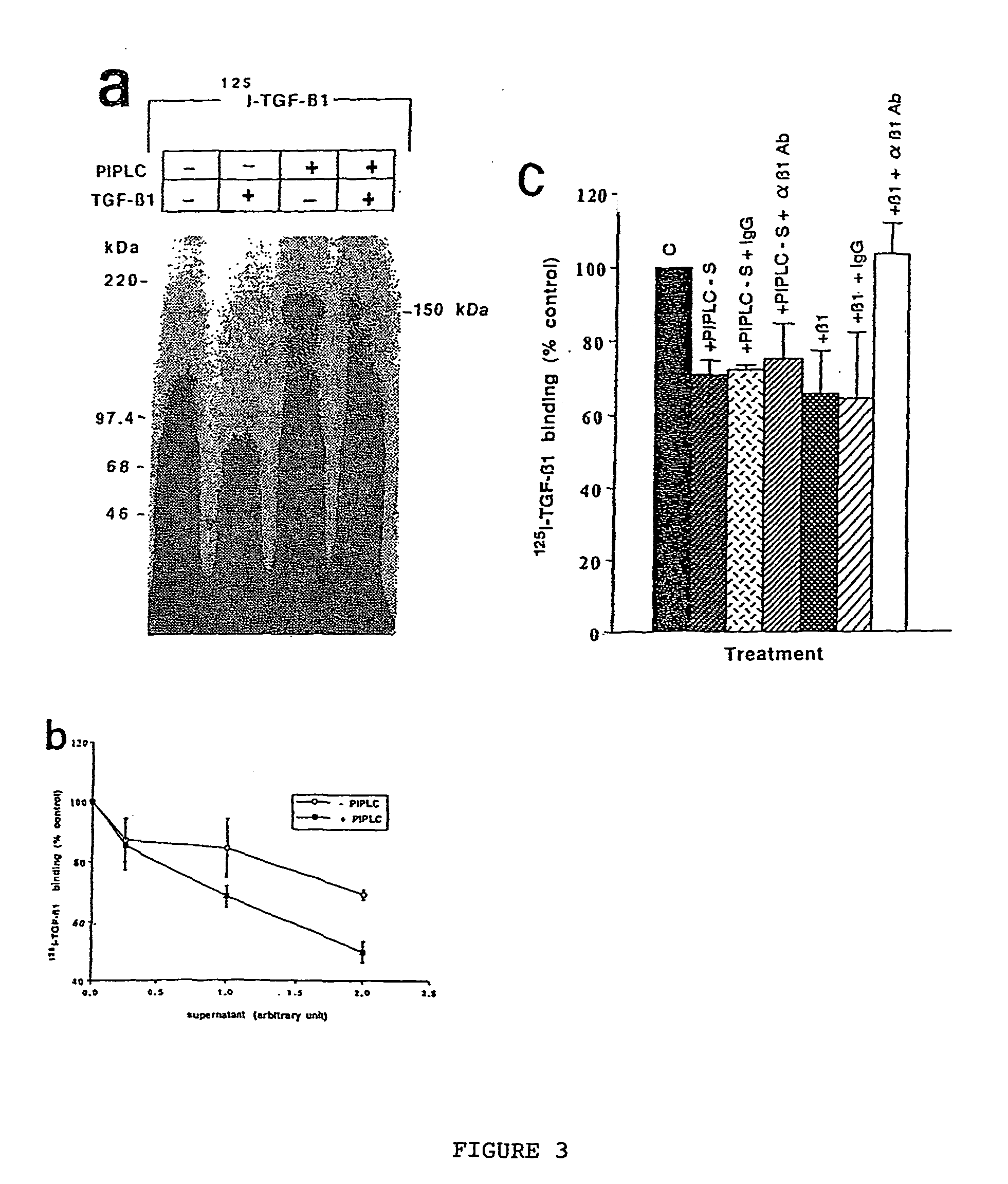
The present invention relates to a TGF-.beta.1 binding protein called r150. This protein has a GPI-anchor contained in r150 itself and not on a tightly associated protein and that it binds TGF-.beta.1 with an affinity comparable to those of the signaling receptors. Furthermore, the released (soluble) form of this protein binds TGF-.beta.1 independent of the types I and II receptors. Also, the soluble form inhibits the binding of TGF-.beta. to its receptor. In addition, evidence that r150 is released from the cell surface by an endogenous phospholipase C is provided. Also, the creation of a mutant human keratinocyte cell line with a defect in GPI synthesis which displays reduced expression of r150 is described. Our results using these mutant keratinocytes suggest that the membrane anchored form of r150 is a negative modulator of TGF-beta responses. These findings, taken together with the observation that r150 forms a heteromeric complex with the signaling receptors, suggest that this accessory receptor in either its membrane anchored or soluble form may antagonize TGF-.beta. responses in human keratinocytes. Experiments with mutants confirmed that TGF.beta.1 activity can be modulated when the expression of the accessory receptor r150 is silenced. The complete nucleic acid and deduced amino acid sequences are now provided. The r150 cloned nucleic acid was used to study overexpression of r150. When r150 gene is overexpressed, TGF.beta. responses are increased. r150 and its derivatives or precursors (fragments, variants and nucleic acids encoding the same) will find a broad clinical utility, knowing that TGF.beta.1 is an important cytokine.
Owner:9406 2668 QUEBEC INC
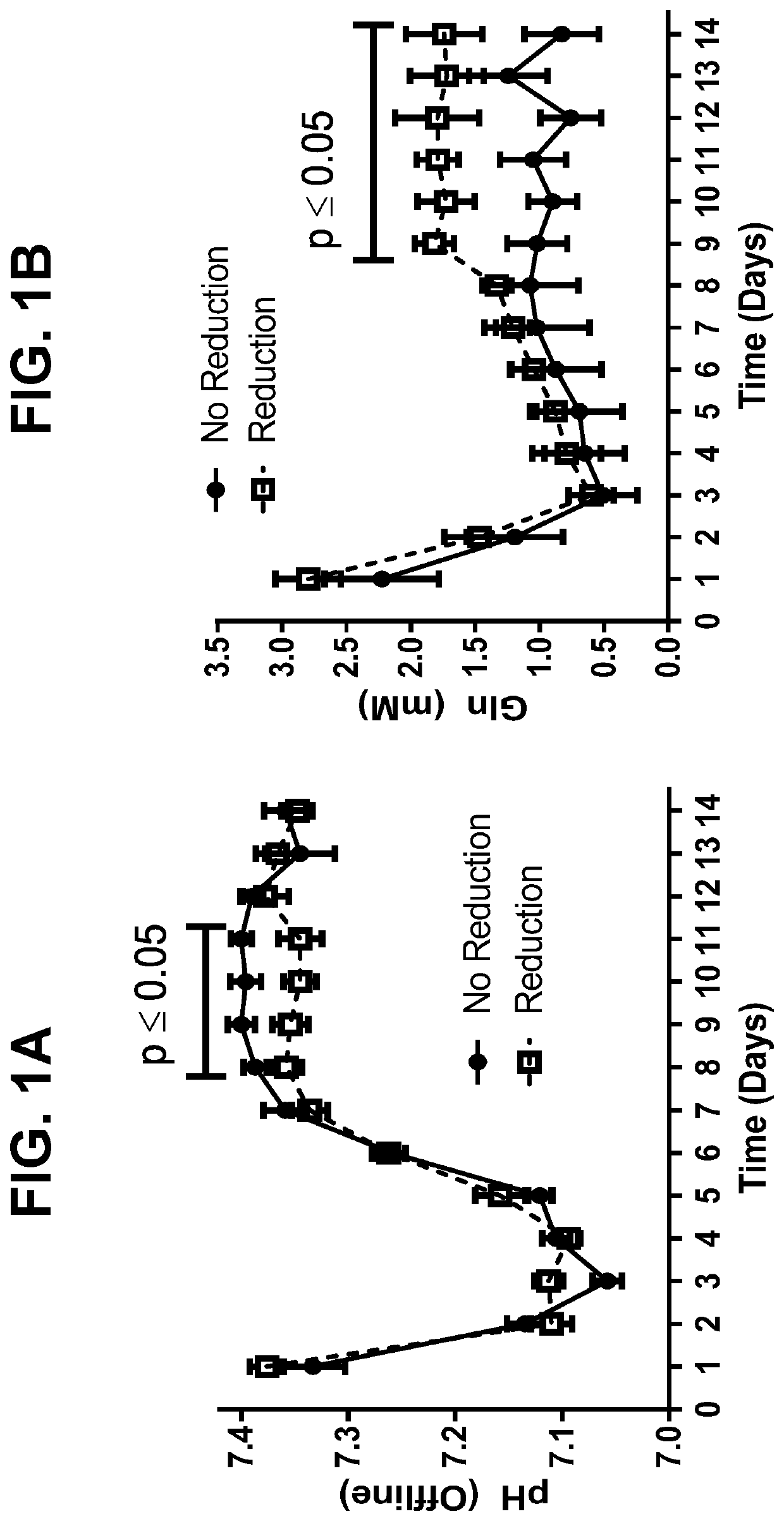
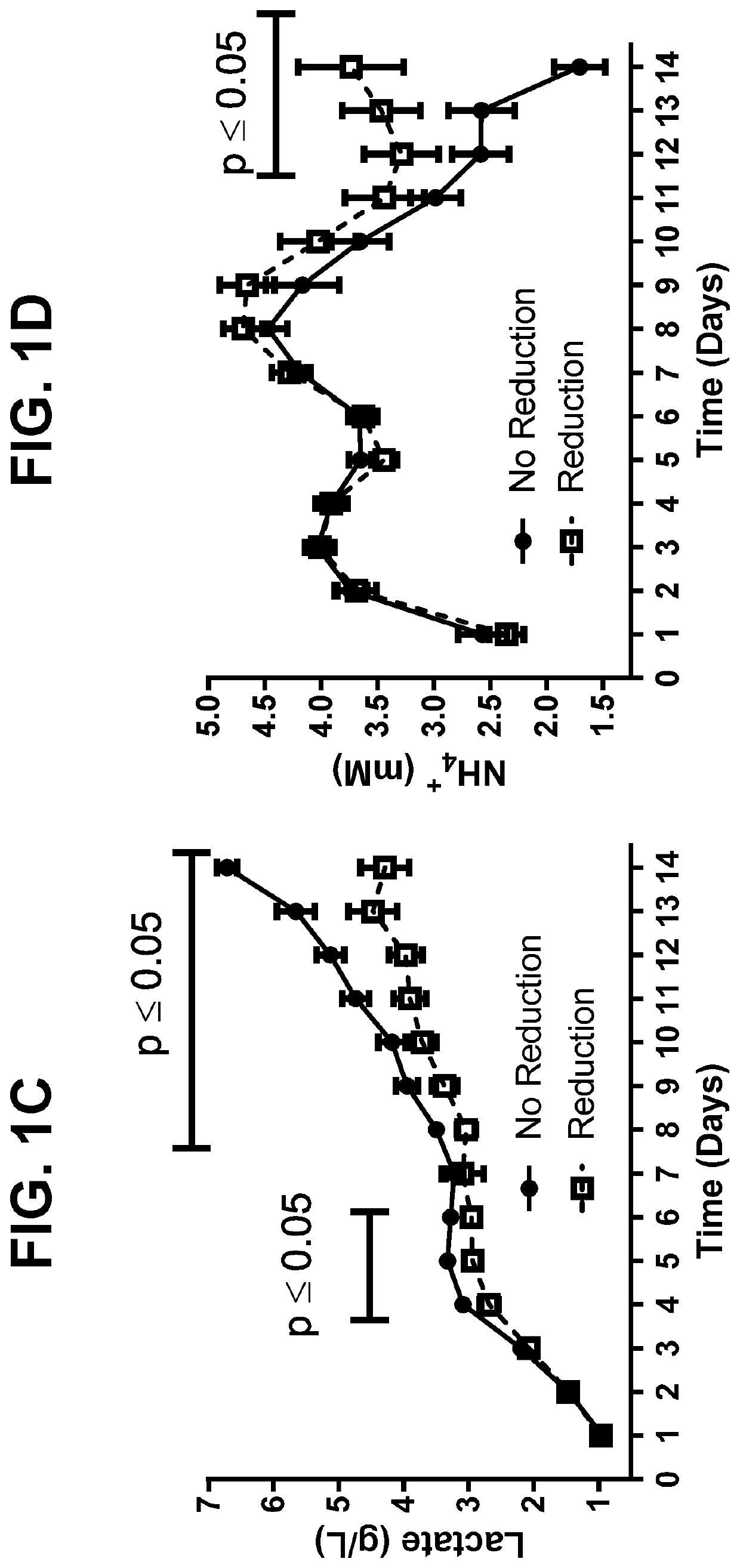
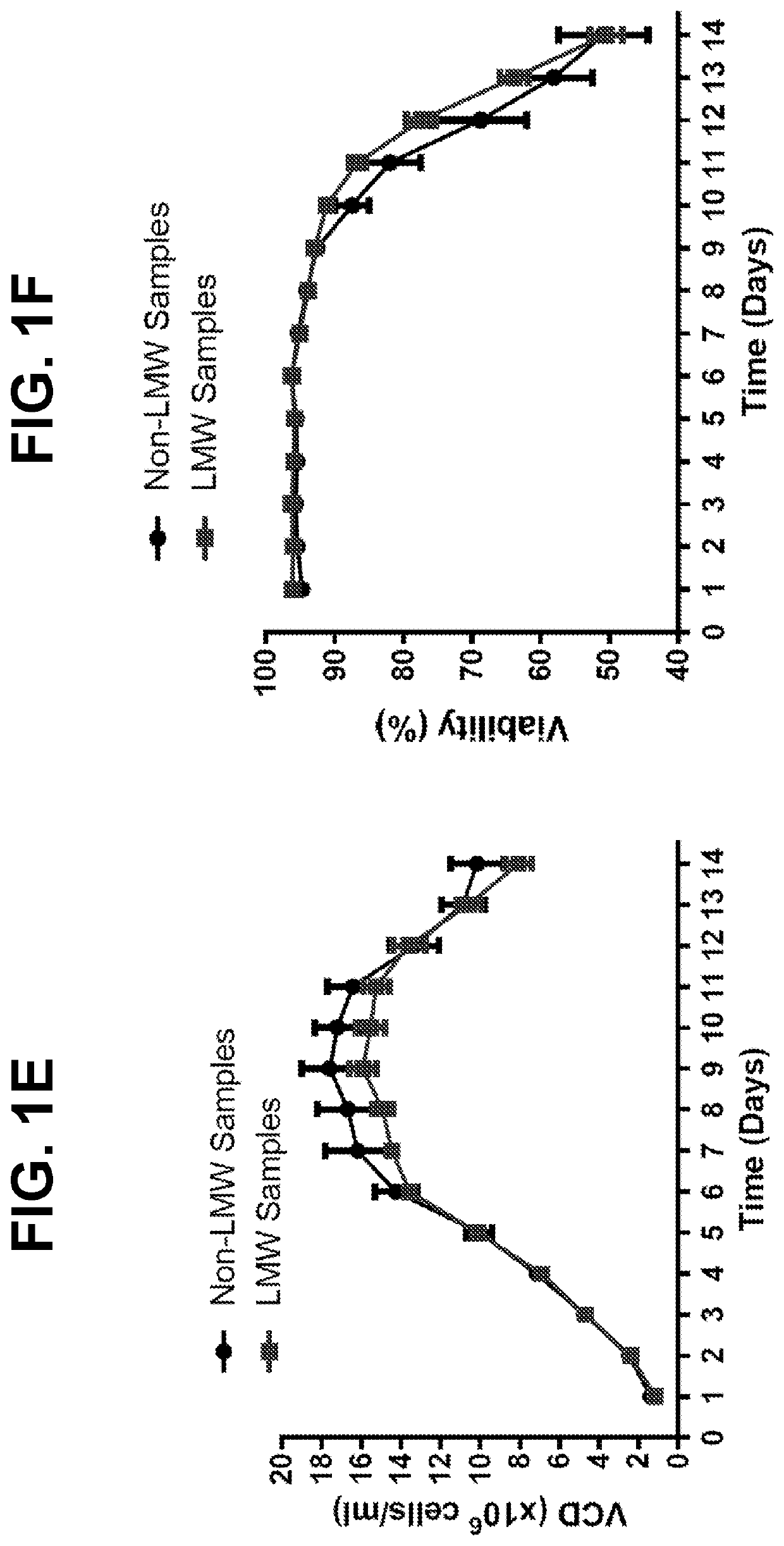
Metabolic enzyme activity and disulfide bond reduction during protein production
PendingUS20210010055A1Microbiological testing/measurementMaterial analysis by electric/magnetic meansDisulfide bondingDisulfide bond reduction
The present disclosure relates to the use of host cell protein biomarkers to assess disulfide bond reduction in compositions comprising a protein of interest. In some embodiments, the disclosure relates to methods of predicting the occurrence of disulfide bond reduction or low molecular weight protein species in compositions comprising a protein of interest, wherein the expression or activity level of at least one host cell protein is measured and provides a benchmark value associated with the occurrence of disulfide bond reduction or low molecular weight species of said protein of interest. In some embodiments, the disclosure relates to methods of producing a protein of interest, wherein host cells capable of producing the protein of interest are cultured, the expression or activity level of at least one host cell protein is measured, and downstream isolation of the protein of interest is informed by the host cell protein measurements.
Owner:BRISTOL MYERS SQUIBB CO
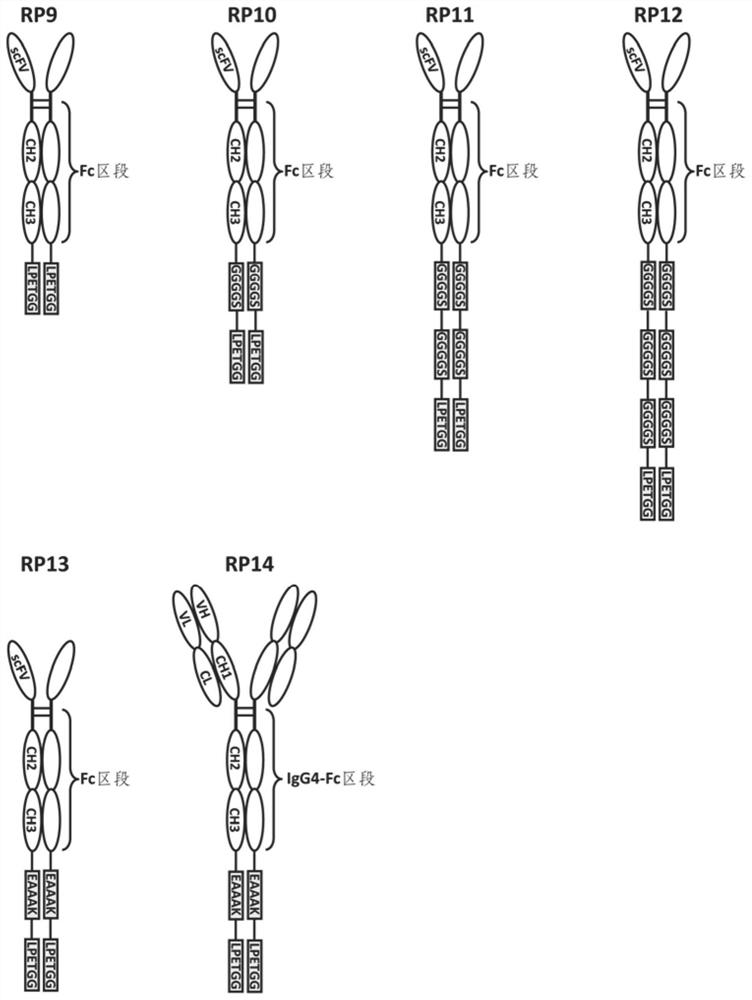
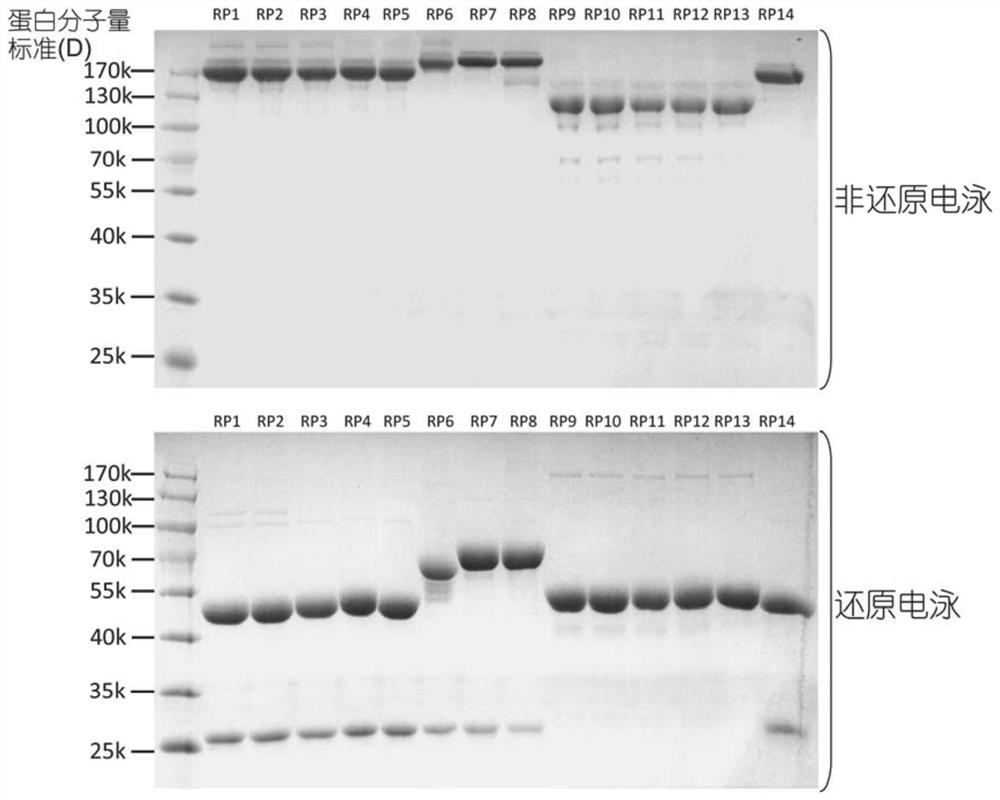
Fusion protein and use thereof
ActiveCN111848816APrecise targetingEasy to prepareCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsESA ProteinEffector cell
The invention relates to a fusion protein and use thereof. The fusion protein comprises, from N to C terminals, a first moiety, an Fc segment, a linker moiety comprising a moiety selected from the group consisting of a linker and a protein or polypeptide selected from the group consisting of IL2 or scFv, and a substrate moiety of transpeptidase A; the linker comprises a sequence selected from thegroup consisting of (1) (GGGGS) n, wherein when the linker moiety comprises the protein or polypeptide and linker, n>=1; when the linker moiety only comprises the joint, n>=3; and (2) (EAAK) n, n >= 1; the substrate moiety comprises a sequence as shown in LPXTG. The fusion protein can be directly connected to cells to enable the cells to have targeting property, is simpler than an existing methodfor preparing targeting cells through cell transfection, and can also reduce the risk possibly generated by effector cell genome operation at the same time.
Owner:SUPERMAB (BEIJING) BIOTECH CO LTD
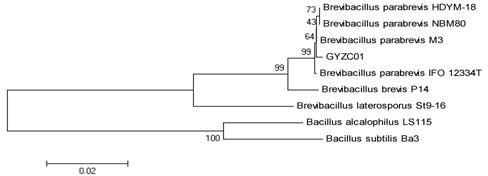
Brevibacillus parabrevis as well as method and application of protein series prepared thereby
InactiveCN102643762AHigh activityLasting effectBacteriaPeptide/protein ingredientsESA ProteinBiochemistry
The invention discloses brevibacillus parabrevis as well as a method and application of a protein series prepared thereby. The classification is named as brevibacillus parabrevis GYZC01, CCTCC NO:M2011461; 16SrRNA of the brevibacillus parabrevis has a sequence of SEQ ID NO:1; the protein series prepared by the brevibacillu sparabrevis GYZC01 strain can be used for preparing thrombolytic medicine, can be used for slowly dissolving thrombus and has durability (EC50 being 200), and can be used for effectively dissolving fresh thrombus (EC50 being 400); and aiming at old thrombus, the activity ofGYZC01 strain crude protein is obviously stronger than that of urokinase (EC50 being 800).
Owner:GUIZHOU UNIV
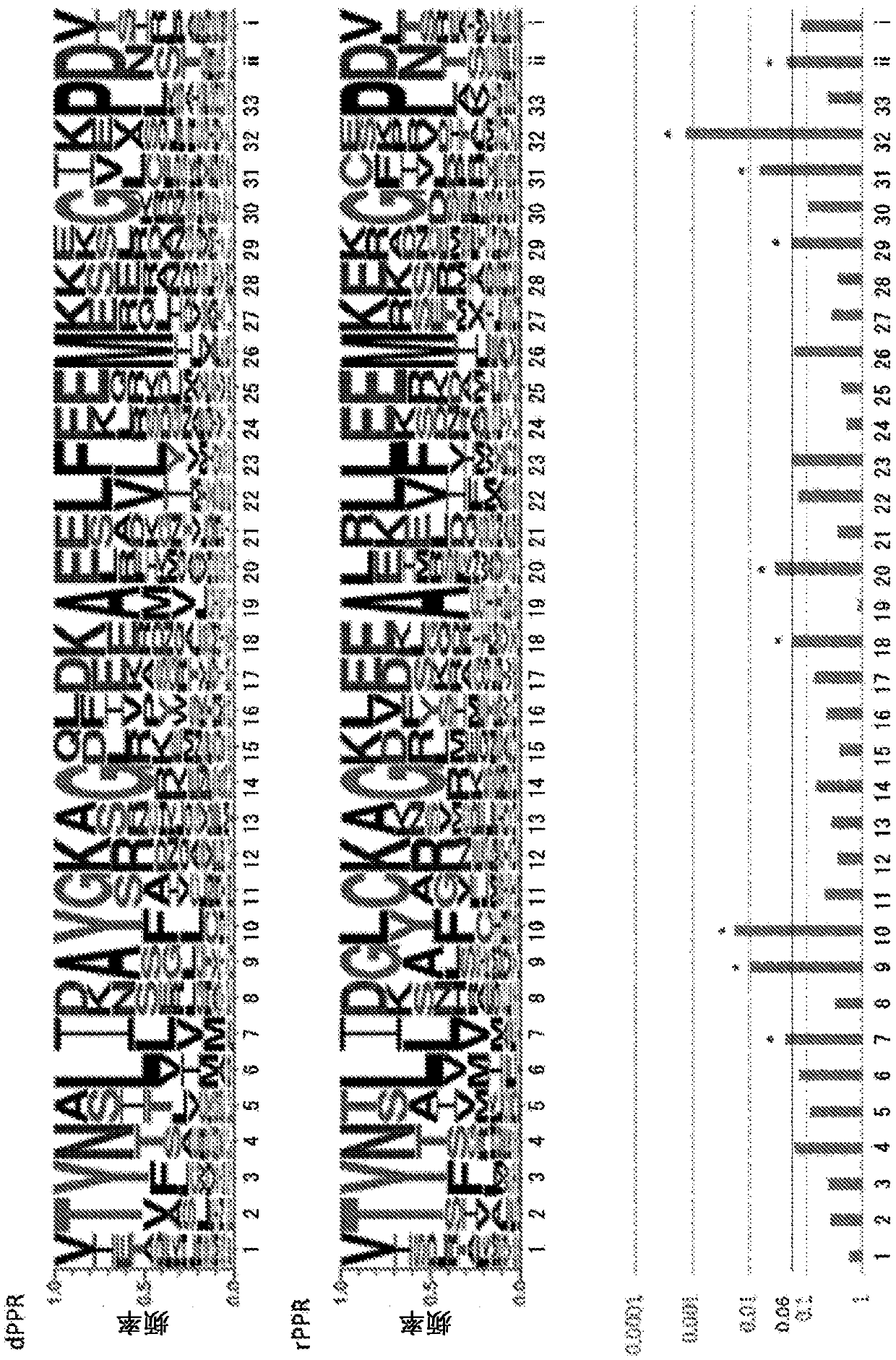
DNA-binding protein using PPR motif and use of said DNA-binding protein
InactiveCN109563137ARealize editingHave binding activityFusion with DNA-binding domainHydrolasesBase JArginine
The present invention addresses the problem of generalizing and improving a DNA-binding protein in which a PPR is used. Provided is a protein including at least one PPR motif having a structure represented by formula (1), wherein one PPR motif (Mn) included in the protein is a PPR motif having three amino acids of No. 1 A.A., No. 4 A.A., and No. ''ii'' (-2) A.A., in a combination of specific aminoacids corresponding to a target DNA base or a target DNA base sequence, and satisfies at least one selected from the group consisting of the following (a)-(h): (a) No. 7 A.A. in the PPR motif (Mn) isisoleucine (I); (b) No. 9 A.A. in the PPR motif (Mn) is alanine (A); (c) No. 10 A.A. in the PPR motif (Mn) is tyrosine (Y); (d) No. 18 A.A. in the PPR motif (Mn) is lysine (K), arginine (R), or histidine (H); (e) No. 20 A.A. in the PPR motif (Mn) is glutamic acid (E) or aspartic acid (D); (f) No. 29 A.A. in the PPR motif (Mn) is glutamic acid (E) or aspartic acid (D); (g) No. 31 A.A. in the PPR motif (Mn) is isoleucine (I); and (h) No. 32 A.A. in the PPR motif (Mn) is lysine (K), arginine (R), or histidine (H). (helix A)-X-(helix B)-L formula (1).
Owner:FUJIFILM WAKO PURE CHEM CORP +1
Protein with cadmium combination performance, and encoding gene and applications thereof
InactiveCN106543275AImprove accumulation abilityEnhanced Cd tolerancePlant peptidesFermentationAgricultural scienceTransgene
The invention discloses a protein with cadmium combination performance, and the encoding gene and applications thereof. The protein is one randomly selected from following proteins: I, a protein with a sequence represented by the 22th to the 110th sits of sequence 1 or sequence 2 in a sequence table; II, a protein with a sequence represented by the 1th to the 113th sits of sequence 1 or sequence 2 in the sequence table; III, a protein with a sequence represented by sequence 3 in the sequence table; and IV, a protein with a sequence represented by sequence 4 in the sequence table, or a protein with a sequence represented by sequence 4 after base deletion of the 116th to the 354th sites. The encoding gene of the protein is transferred arabidopsis thaliana via genetic engineering technology, so that accumulation ability of the overground part of arabidopsis thaliana on heavy metal Cd is increased obviously, and Cd tolerance of obtained transgenic plants is increased obviously. The above gene transferring method is high in practicability, can be used for obtaining plants which possess heavy metal tolerance and heavy metal ion accumulation ability, and can be planted in heavy metal polluted soil, and obtaining novel plant varieties used for soil restoration.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Bioactive Protein, Use Thereof and Method for its Production
InactiveUS20170204147A1Weight increaseDry weight increaseBiocideDepsipeptidesBiotechnologyESA Protein
The present invention according to a firrst aspect relates to a Trichoderma longibrachiatum strain comprising an autologous nucleotide sequence coding for a protein having at least 70% sequence similarity with the amino acid sequence of SEQ ID NO: 1 and its use for the treatment of a plant. Further aspects of the invention relate to a protein derived from said strain, methods for producing the protein, the use of the protein for the treatent of a plant and compositions comprising a source of the protein of the invention. Yet Further aspects of the invention relate to seeds treated with a source of the protein and methods for growing plants incorporating the step of treating said plants with the composition of the invention.
Owner:KOPPERT
Protein capable of improving salt tolerance and drought tolerance of plants as well as coding gene and application of protein
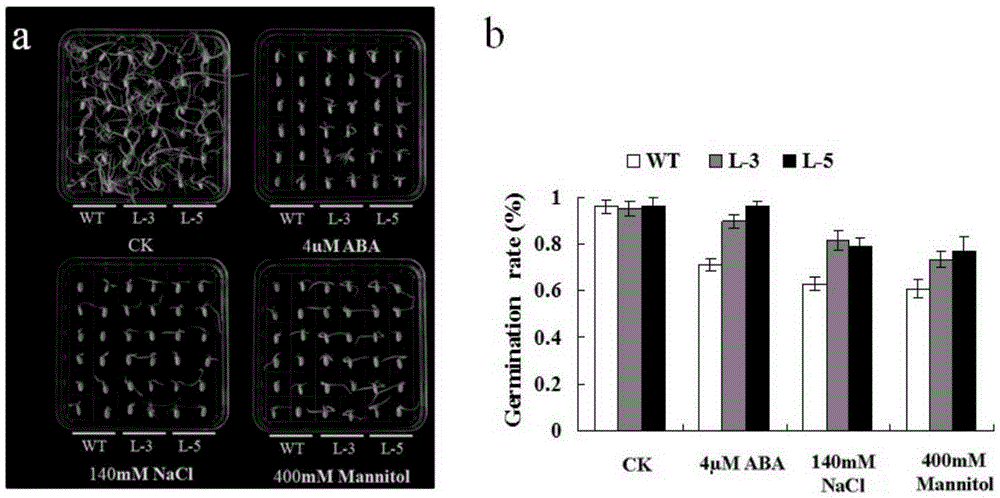
InactiveCN105254730AImprove germination rateImprove survival ratePlant peptidesFermentationCell membraneMannitol
The invention discloses a protein capable of improving salt tolerance and drought tolerance of plants as well as a coding gene and application of the protein. The protein has a sequence represented by SEQ ID NO.1 and is named as MePMP3-2. The coding gene of the protein has a sequence represented by SEQ ID NO.2 and is named as gene MePMP3-2. The protein coded by the gene MePMP3-2 is positioned in cell membranes of plants, and the coding gene MePMP3-2 of the protein is introduced into the plants, so that the germination rate of seeds of the transgenic plants under the treatment of ABA, NaCl and mannitol is increased, the root lengths and the seedling lengths of the transgenic plants under high-salinity and high-mannitol under adversities are increased, and the transgenic plants are relatively high in survival rate, relatively low in malondialdehyde (MDA) content and relatively high in proline (Pro) content under the stress of drought and high salinity; the relative expression quantities of adversity relevant genes including OsProT, OsP5CS, OsDREB2A, OsLEA3 and the like in the transgenic plants are increased at different degrees under adversity stress.
Owner:山西尧山种业科技有限公司
Enhancing ingredients for protein production from various cells
InactiveUS20140162318A1Increase productionIncrease volumeCell culture mediaPeptidesBiotechnologyAdditive ingredient
[Summary] The present invention relates to a protein production accelerating agent that has enabled to largely increase the produced amount of a desired protein by adding polysaccharides to a medium for animal cells containing a serum or serum alternative, and a production method of a protein using a medium containing the protein production accelerating agent.
Owner:NISSAN CHEM IND LTD +1
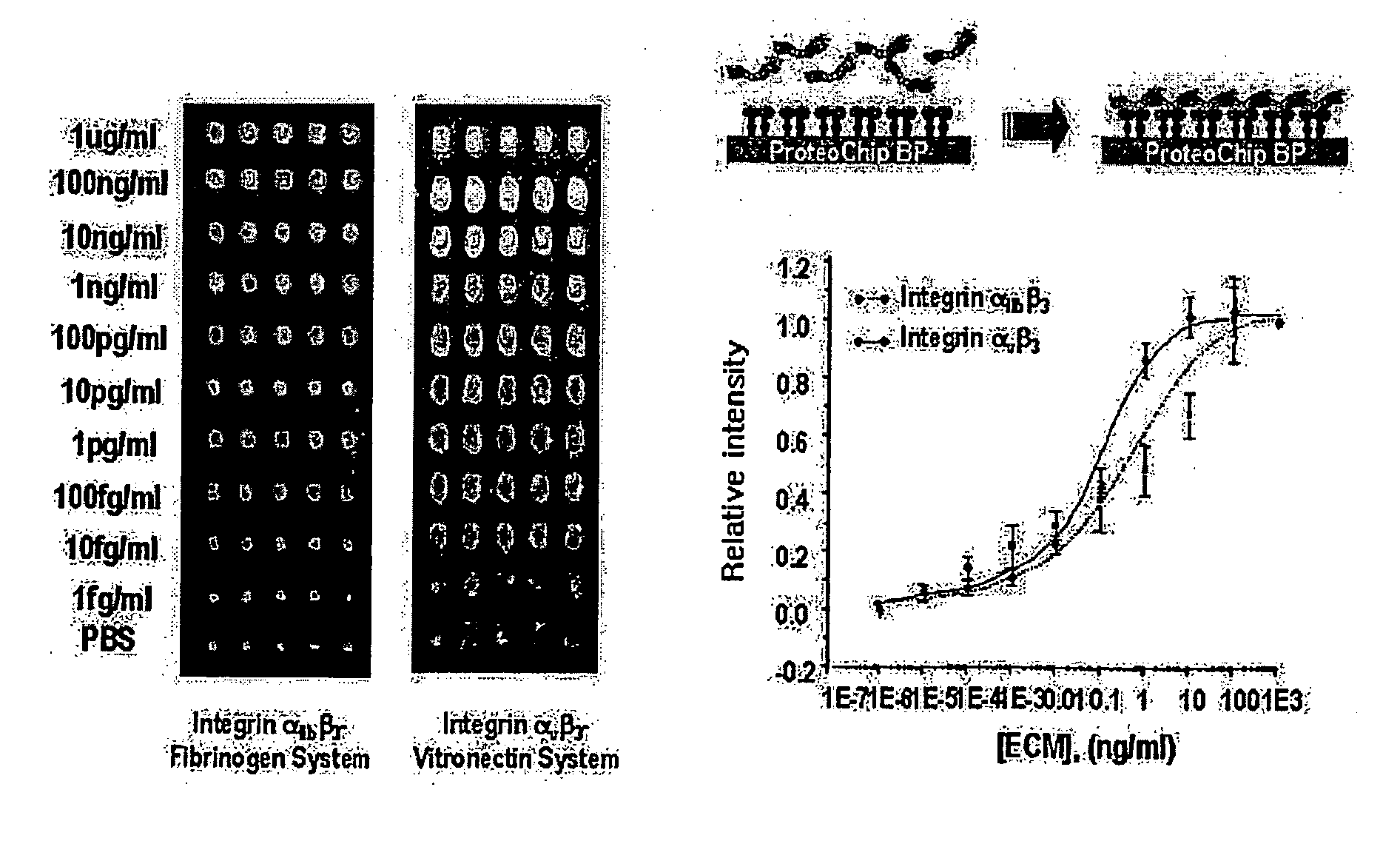
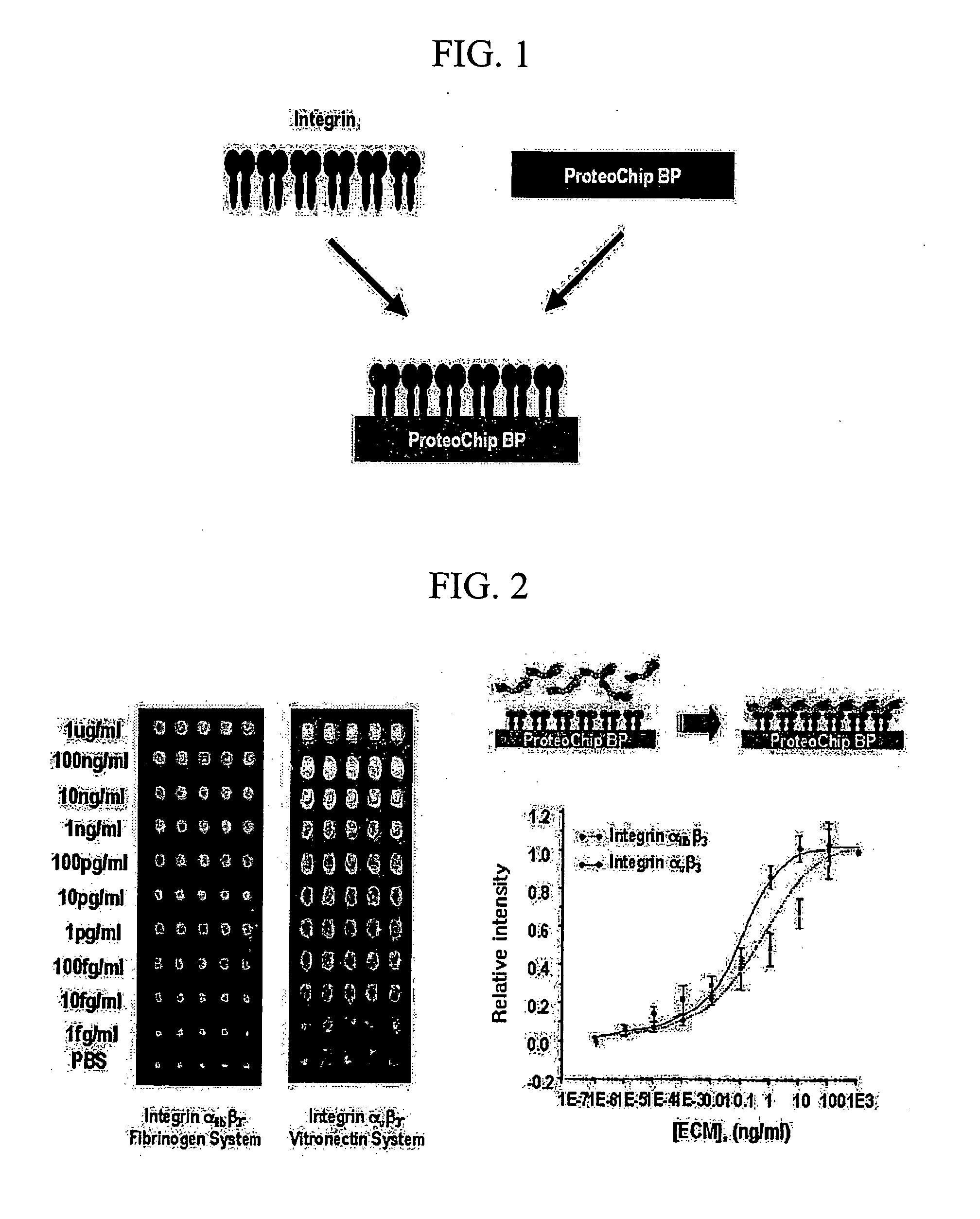
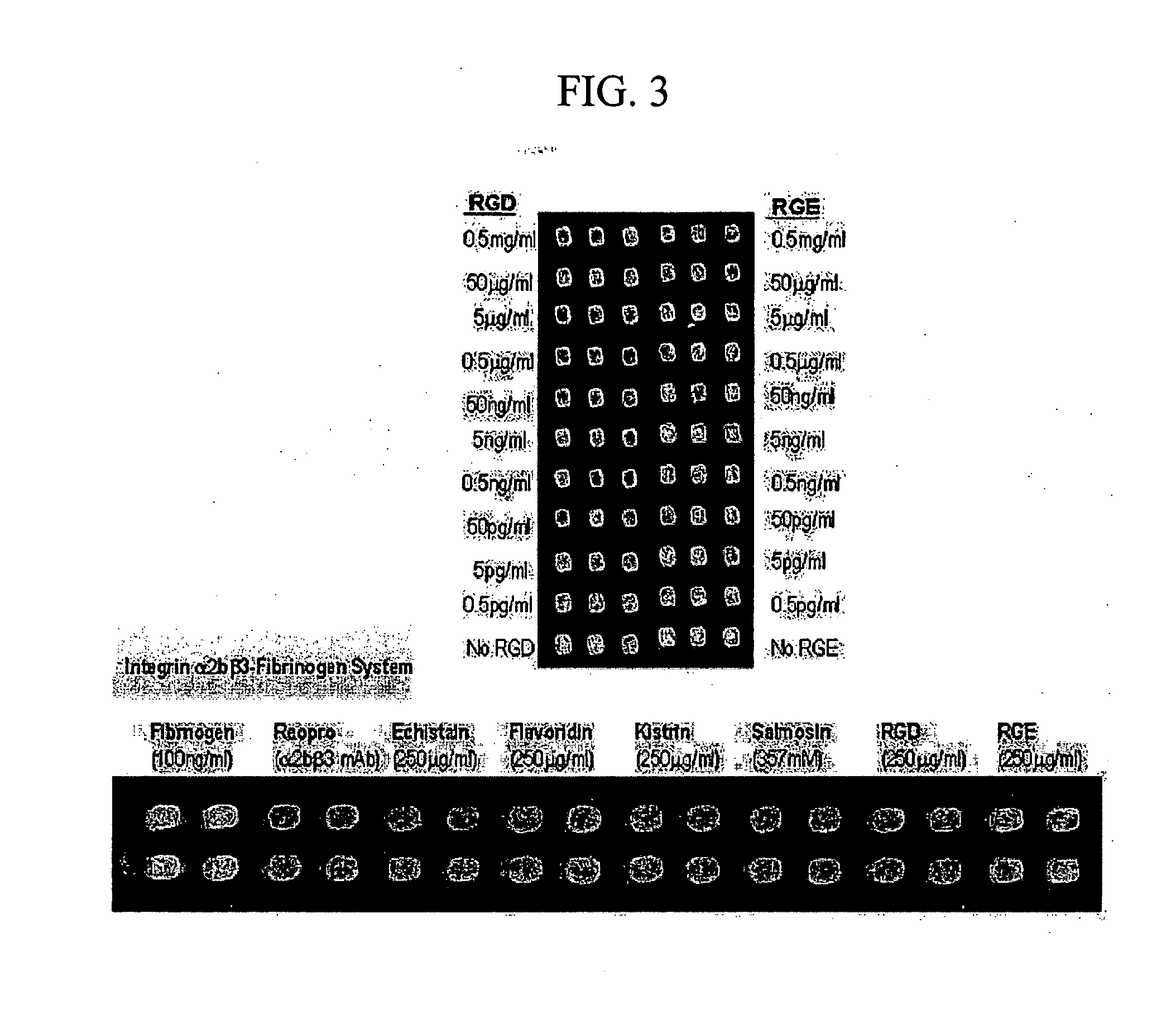
High-Throughput Screening Method for Intergrin Antagonist and New Peptide Screened Therefrom
InactiveUS20070249061A1Bioreactor/fermenter combinationsPeptide/protein ingredientsCrystallographyHigh-Throughput Screening Methods
The present invention relates to the screening method of antagonistic material of integrin using the protein chip and useful peptides screened thereby. The protein chip used in the present invention is unique substrate coated with new material, calixarene derivative, which can keep uniform and high activities of proteins. Integrin receptor protein is arrayed high densely on the chip, and materials (protein, peptide, small molecules and so on) specifically inhibiting the binding of ligand can be screened therewith. The integrins used in the present invention are integrin av f3 3 and integrin a1, b j3 3, and new antagonistic peptides screened from peptide library have high binding affinity.
Owner:PROTEOGEN
Novel protein and gene encoding the same
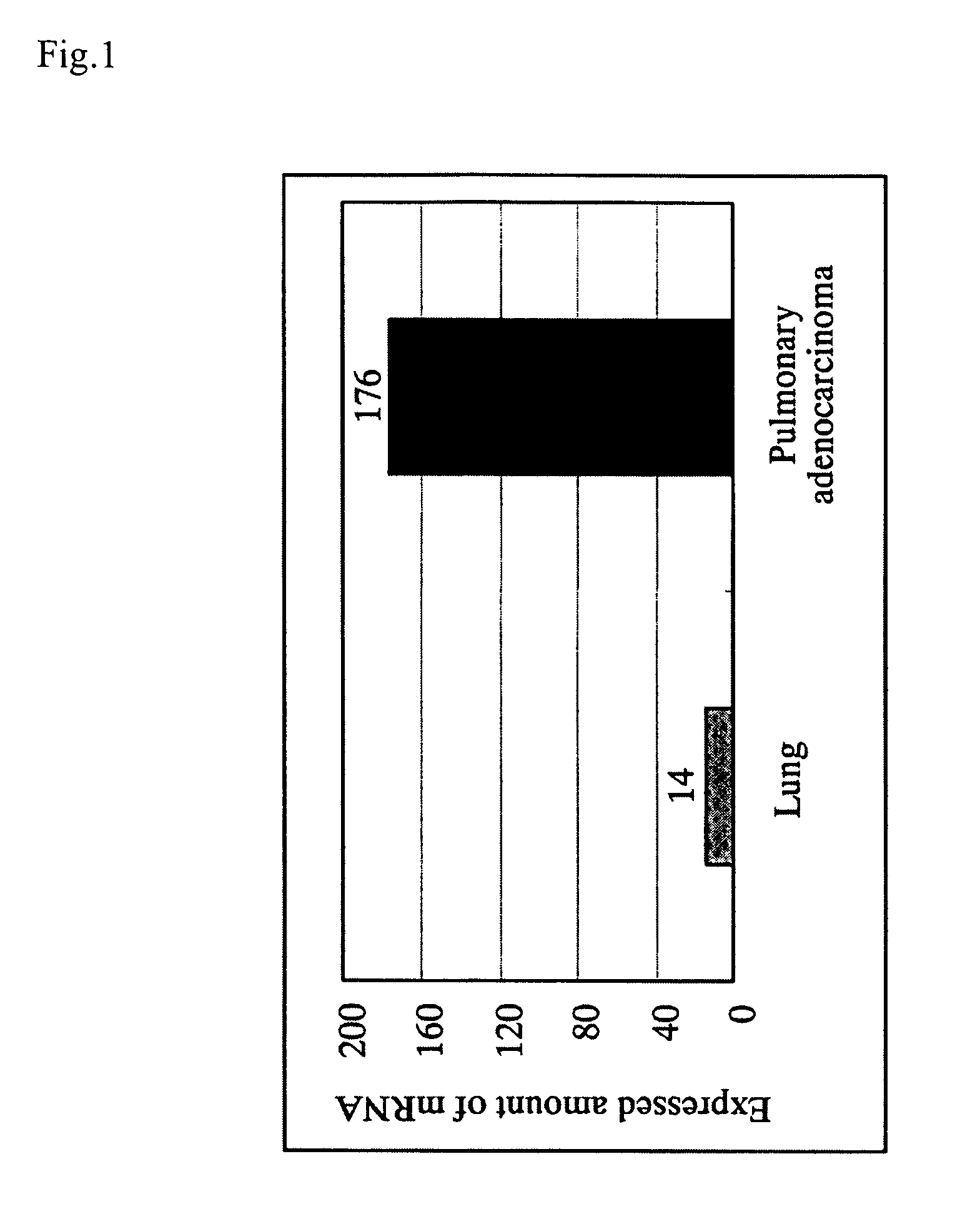
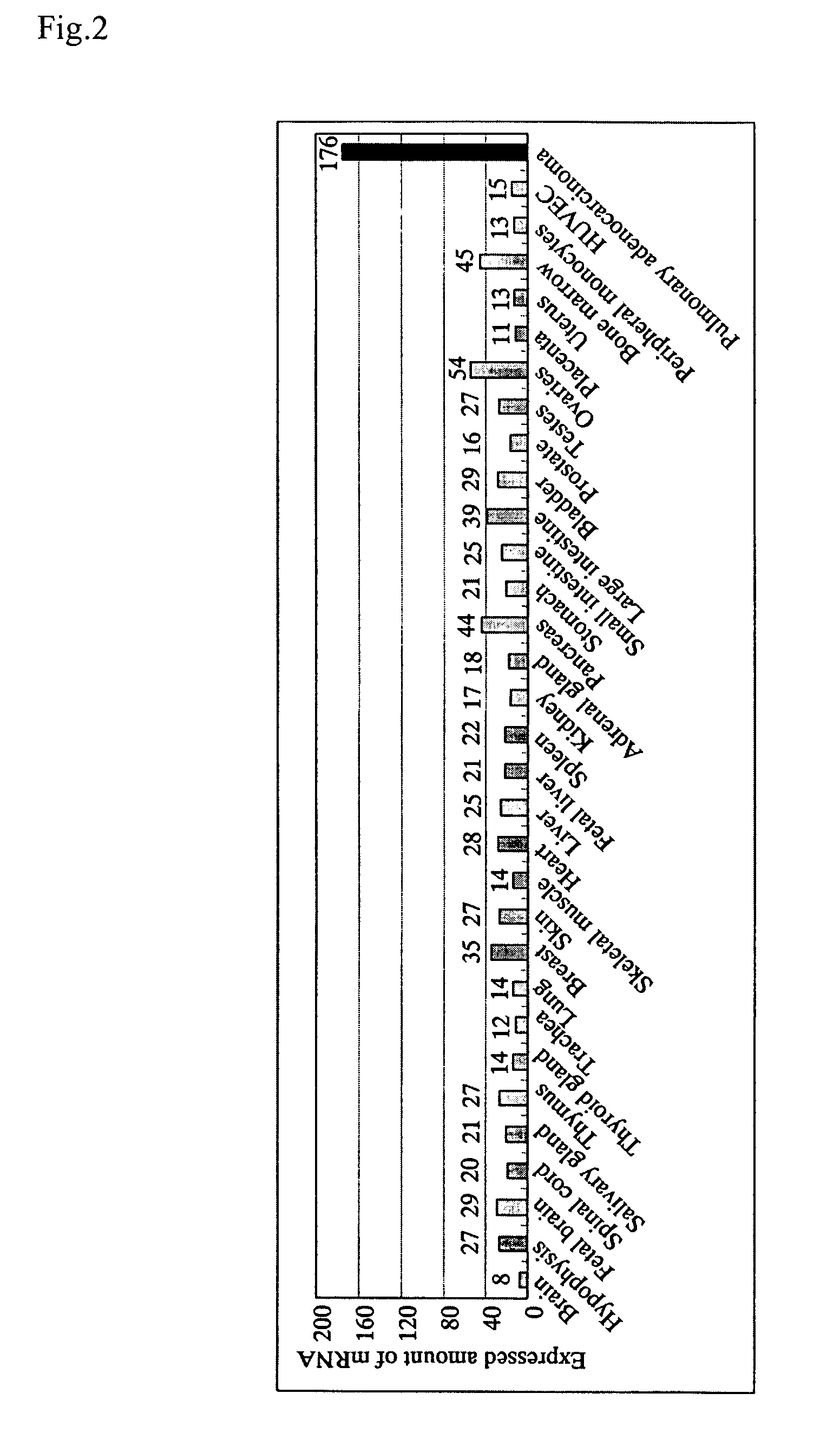
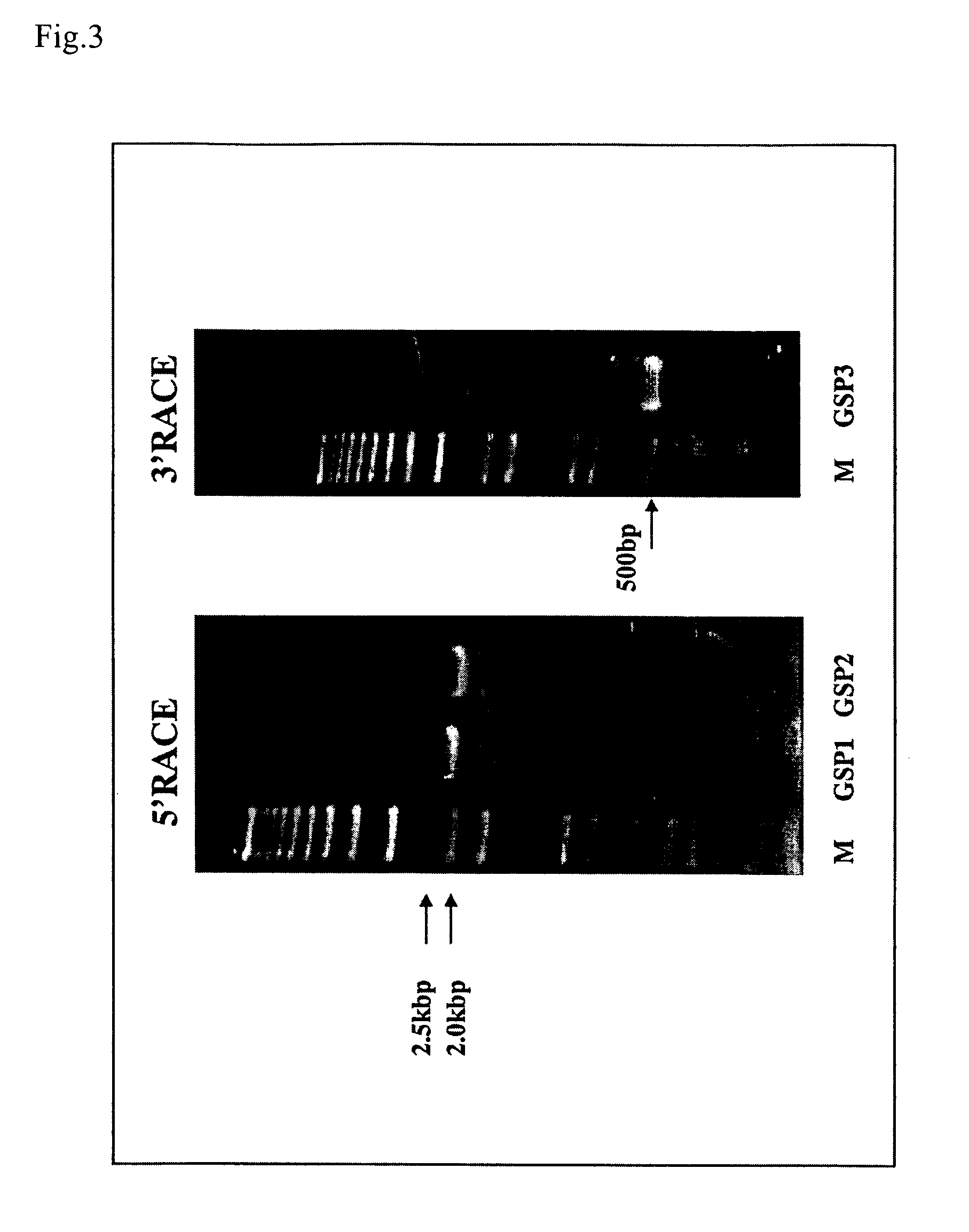
InactiveUS20060135750A1Guaranteed flatnessCell receptors/surface-antigens/surface-determinantsSugar derivativesGene expression levelAbnormal cells
With the object of providing a novel protein expression of which is specifically elevated in abnormal cells or abnormal tissue, and also providing a diagnostic method and diagnostic kit for diseases involving elevated expression of a gene encoding that protein, along with a screening method and screening kit for substances for preventing and treating diseases involving elevated expression of a gene encoding that protein, (a) a protein comprising the amino acid sequence represented by Seq. ID No. 2 or (b) a protein comprising the amino acid sequence represented by Seq. ID No. 2 with one or more amino acids deleted replaced or added is provided as a novel protein expression of which is specifically elevated in abnormal cells or abnormal tissue, with a protein expression of which is specifically elevated in abnormal cells or abnormal tissue, the level of expression of a gene encoding that protein is taken as a marker for diagnosing a disease and a reduction effect on the level of expression of a gene encoding that protein is taken as a marker for screening preventative and therapeutic substances for a disease.
Owner:TOUDAITLO LTD +1
Humanized transgenic single nucleotide polymorphism animal systems
The present invention relates to the field of transgenic animals. More specifically, the present invention provides methods and composition related to humanized transgenic single polymorphism non-human animal systems. In one embodiment, a system comprises (a) a transgenic non-human animal comprising a transgene encoding a wildtype human protein, wherein the protein is biologically active in the animal; and (b) at least one transgenic non-human animal comprising a transgene encoding a variant human protein, wherein the protein is biologically active in the animal and wherein the variant comprises one or more single nucleotide polymorphisms (SNPs).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods, uses and kits for monitoring or predicting response to peripheral disease treatment
An in vitro method for assessing or predicting the response of a human patient to periodontal disease treatment is disclosed. The method is based on determining insights to biomarker proteins. Accordingly, the concentration of a specific protein combination is measured in a saliva sample of a patient. One such combination is at least one of interleukin 1 [beta] (IL1 [beta]) and matrix metalloproteinase 8 (MMP8), and [alpha] 1 acid glycoprotein (A1AGP). At least one value reflecting the combined concentration of the protein is determined based on the measured concentration. The at least one value may indicate a likelihood that the human patient has or will successfully treat periodontitis. The at least one value may be compared to at least one threshold that in the same manner reflects a combined concentration associated with successful treatment of periodontitis. The comparison allows evaluation of whether the test value is an indication of the patient's periodontal treatment status.
Owner:KONINKLJIJKE PHILIPS NV
Novel protein and gene that codes therefor
Owner:JAPAN TOBACCO INC
Method for improving repebody containing repeat modules
InactiveUS20150133308A1High binding affinityIncreased specificity and activityPolypeptide with localisation/targeting motifPeptide-nucleic acidsBioinformaticsProtein
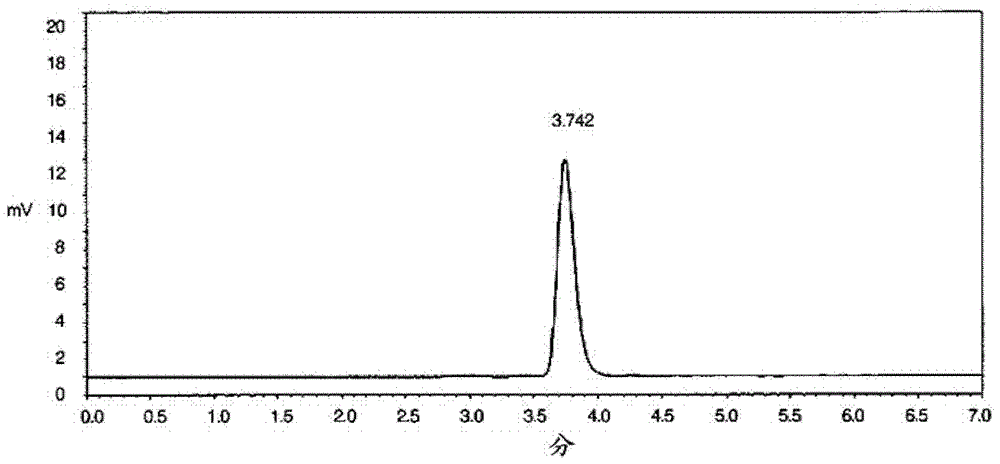
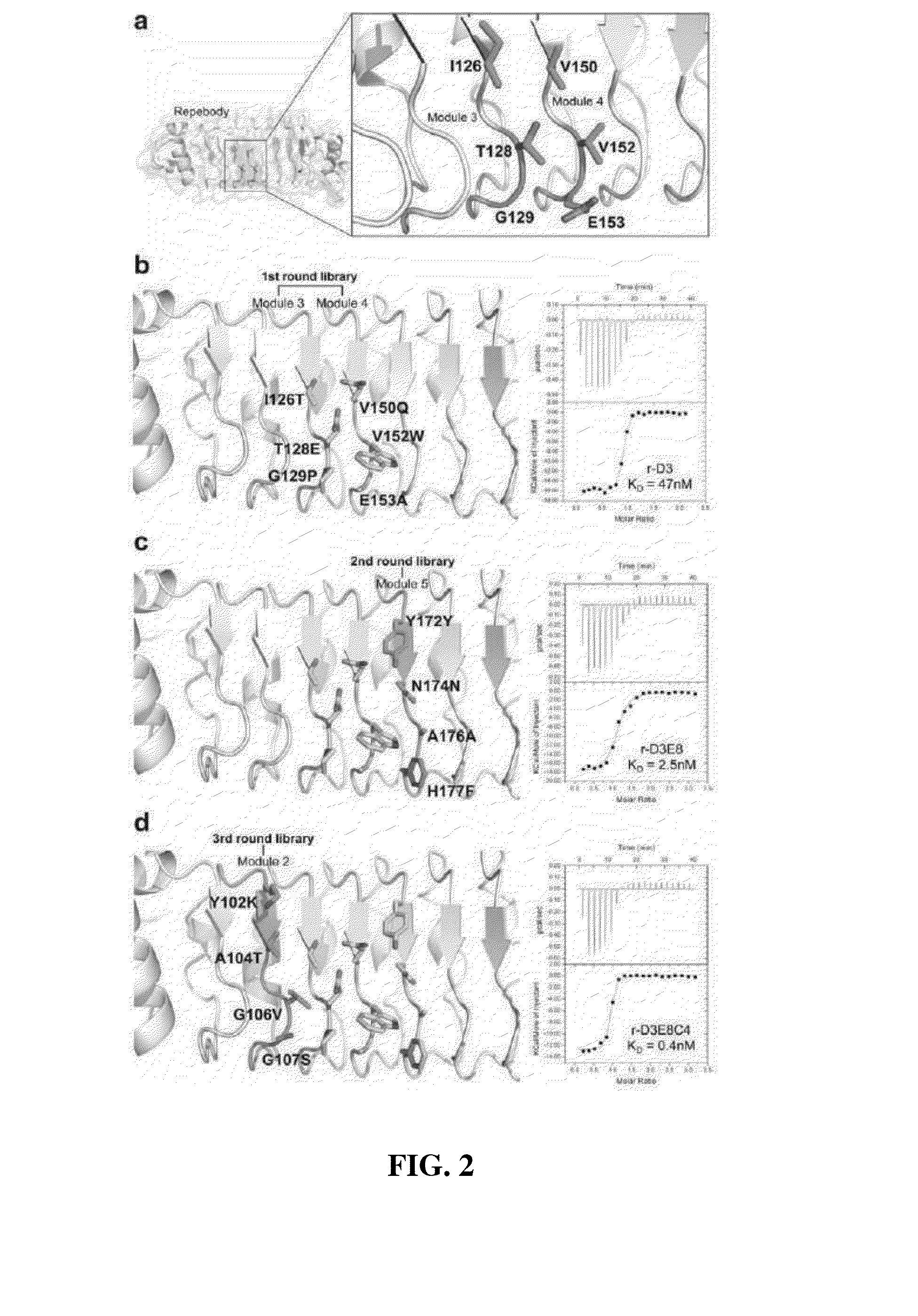
The present invention relates to a method for improving a repebody protein comprising repeat modules and a nucleotide library encoding a repebody protein library for improving the repebody protein. More particularly, the present invention relates to a method for improving a repebody protein using a module evolution method of sequentially mutating repeat modules constituting the repebody protein, and a nucleotide library encoding a repebody protein library used to improve the protein. According to the module evolution method of the present invention, an improved repebody protein can be screened which has a high binding affinity and accordingly increased specificity and activity, and thus it is easy to express a repebody used as an inhibitor, a therapeutic agent, and an analysis means against a target substance.
Owner:KOREA ADVANCED INST OF SCI & TECH +2
Stable immunogenic protein having multiple cysteines molecules process therefor and composition thereof
InactiveUS20120269856A1Increased purity and stability and immunogenicityWithout impairing antigen stability and integrity and functionalityPeptide preparation methodsDepsipeptidesAdjuvantInclusion bodies
The invention describes a stable immunogenic protein having multiple cysteines molecules wherein the protein is having stability up to two years and purity more than 98% particularly rPvRII and / or rPfF2. It also discloses a method for producing said immunogenic protein comprising the following steps: culturing the host E. coli cells containing a desired recombinant gene construct comprising a codon optimized gene sequence of rPvRII and / or rPfF2 to produce cells in high density; inducing expression rPvRII and / or rPfF2 as inclusion bodies; harvesting the cells and isolating the said inclusion bodies; separating rPvRII and / or rPfF2 from inclusion bodies by repeated sequential washing and solubilizing with chaotrophic agents comprising guanidine hydrochloride and / or urea; purifying the protein by subjecting to metal-chelate affinity chromatography; re-folding of the purified rPvRII and / or rPfF2 obtained in step e) with a redox system to recover a high yield of the soluble protein, followed by further purifying the desired protein by removing impurities by subjecting to chromatography. Further the invention discloses formulation comprising rPvRII or rPfF2, preferably being lyophilized using polysaccharides preferably sucrose, lactose, and pharmaceutically acceptable adjuvants such as aluminum hydroxide, aluminum phosphate, CpG nucleotides, non-CpG nucleotides, Montanide ISA-720, MF-59, Mono-phosphoryl Lipid-A (MPL-A) and QS-21.
Owner:BHARAT BIOTECH INTERNATIONAL
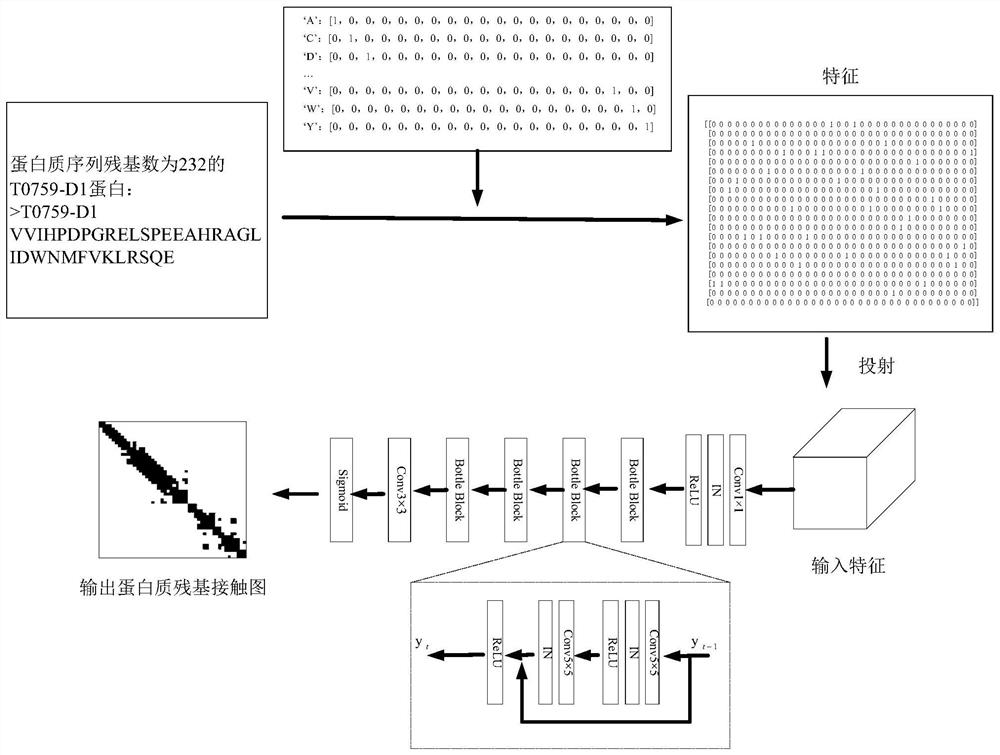
Protein residue contact diagram prediction method based on deep residual neural network
InactiveCN111667880AImprove forecast accuracyImprove forecasting efficiencyNeural architecturesMolecular structuresData setESA Protein
The invention discloses a protein residue contact diagram prediction method based on a deep residual neural network. The method comprises the following steps: firstly, inputting a protein sequence tobe subjected to protein residue contact diagram prediction; then, converting the protein sequence into a 20 * L matrix by utilizing one-hot representation forms of 20 common amino acids for the protein sequence, digitizing the protein sequence information, and calculating by utilizing the 20 * L matrix to obtain a 20 * L * L covariance tensor, i.e., the characteristics of the input network; secondly, establishing a deep residual neural network framework, collecting protein sequences and tags of existing protein contact tags from a PDB library, calculating feature tensors of the protein sequences, forming a data set by the feature tensors and the corresponding tags, and learning a prediction model on the data set by using the deep residual neural network framework; and finally, inputting the protein characteristic tensor to be subjected to protein residue contact diagram prediction into the model to obtain a protein sequence residue contact diagram. The method is low in calculation costand high in prediction precision.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com