Brevibacillus parabrevis as well as method and application of protein series prepared thereby
A technology of Bacillus brevis and species, which is applied in the field of Bacillus brevis and its prepared protein series and applications, and can solve the problems of short half-life, easy to cause internal bleeding, low specificity of thrombolysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
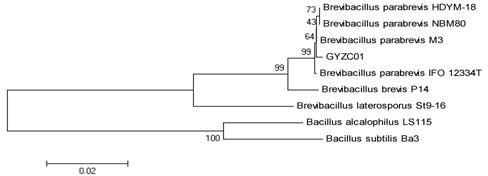
[0022] Example 1: Determination of the 16S rRNA sequence of Bacillus brevius GYZC01
[0023] 1. Genome extraction and electrophoresis detection
[0024] 1. Genome extraction process:
[0025] Extract according to the instructions of Sangon SK1201-UNIQ-10 Column Bacterial Genomic DNA Extraction Kit.
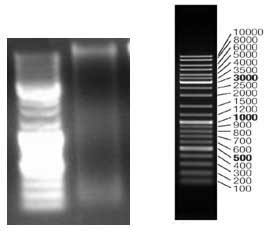
[0026] 2. Genome electrophoresis analysis map: see the attached manual figure 2
[0027] 2. PCR reaction
[0028] 1. PCR system establishment (50ul):
[0029] Template (genome) 10pmol
[0030] Primer up (10uM) 1ul
[0031] Primer down (10uM) 1ul
[0032] dNTP mix (10Mm each) 1ul
[0033] 10*Taq reaction buffer 5ul
[0034] Taq (5u / ul) 0.25ul
[0035] Add water to 50ul
[0036] PCR program setting
[0037] Pre-denaturation at 98°C 5mim;
[0038] Cycle 95°C 35S, 55°C 35S, 72°C 1min 30s, 35 cycles, extension 8min.
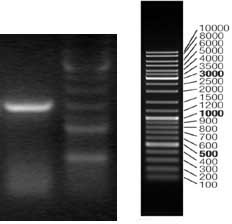
[0039] 3. Electrophoretic pattern of PCR products
[0040] 4. Primer sequence:
[0041] 27f 5'AGAGTTTGATCCTGGCTCAG 3' 20bp
[0042] 1492r 5' GGTTACCT...
Embodiment 2
[0047] Example 2: Preparation of protein series with a molecular weight greater than 7000D of Bacillus brevus strain GYZC01
[0048] 1. Experimental materials
[0049] Brevibacillus parabrevis GYZC01 strain, peptone, sodium chloride, glucose, dialysis bag, ammonium sulfate.
[0050] 2. Experimental method
[0051] 2.1 Bacterial culture
[0052] Composition of bacterial culture medium: peptone 1g, sodium chloride 0.5g, glucose 1.5g, distilled water 100ml, autoclaved for later use. Bacteria were inoculated in 100ml of culture solution and cultured at 30°C for 48h to obtain seeds. According to the ratio of 1 to 100, the seed bacteria solution was added to the culture solution, 5 L was co-cultured, and the culture was statically cultivated at 30°C for 96-120 hours for later use.
[0053] Bacterial fermentation broth (5L) was frozen overnight, thawed at room temperature, and then frozen overnight, and this was repeated 3 times to obtain a frozen lysate, which was centrifuged...
Embodiment 3
[0055] Embodiment 3: In vitro thrombolysis test of Bacillus brevus strain GYZC01 molecular weight greater than 7000D protein series
[0056] 1. Experimental materials
[0057] Crude protein with a molecular weight greater than 7000D of Bacillus brevis GYZC01 strain, capillary, urokinase, plate; 2. Experimental method
[0058] Disinfect the ring finger with alcohol, collect blood by acupuncture, inhale the blood with a capillary, place it horizontally, and the blood will coagulate in about 10 minutes. Dissolve the crude protein of Bacillus brevius GYZC01 strain with normal saline to make sample solutions with series concentrations. Urokinase is used as positive control and normal saline is used as negative control. The above samples, positive control and negative control solutions are placed in 60mm plates respectively. 1. Cut the capillaries with blood clots into 5mm lengths, put them into petri dishes, 5 capillaries per dish, make them immersed in the liquid, put the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com