Fusion protein and use thereof
A fusion protein and protein technology, applied in the field of fusion proteins, can solve the problems of large molecular weight and complex structure of the Fc segment, and achieve the effects of simple preparation method, good safety and reduced risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Expression and purification of transpeptidase A protein
[0077] 1. Construction of recombinant expression plasmid pET-SrtA
[0078] 1. The gene sequence of transpeptidase A is shown in sequence 1, and primer F is designed and synthesized according to sequence 1: 5'-CGGCAGC CATA TG GCTAAACCTCAAATTCCGA-3' (the underline is the restriction endonuclease NdeI digestion recognition sequence, sequence 35) and primer R: 5'-GTGGTG CTCGAG TTATTTGACTTCTGTAGCTAC-3' (underlined is the recognition sequence for restriction endonuclease XhoI, sequence 36).
[0079] Sequence 1:
[0080] ATGGCTAAACCTCAAATTCCGAAAGATAAATCGAAAGTGGCAGGCTATATTGAAATTCCAGATGCTGATATTAAAGAACCAGTATATCCAGGACCAGCAACAAGCGAACAATTAAATAGAGGTGTAAGCTTTGCAGAAGAAAATGAATCACTAGATGATCAAAATATTTCAATTGCAGGACACACTTTCATTGACCGTCCGAACTATCAATTTACAAATCTTAAAGCAGCCAAAAAAGGTAGTATGGTGTACTTTAAAGTTGGTAATGAAACACGTAAGTATAAAATGACAAGTATAAGAAACGTTAAGCCTACAGATGTAGGAGTTCTAGATGAACAAAAAGGTAAAGATAAACAATTAACATTAATTACTTGTGATGATTACAAT...
Embodiment 2
[0096]Example 2: Preparation and binding activity identification of fusion protein containing Fc segment
[0097] 1. Preparation of fusion protein containing Fc segment
[0098] 1. Construction of fusion protein particles containing Fc segment
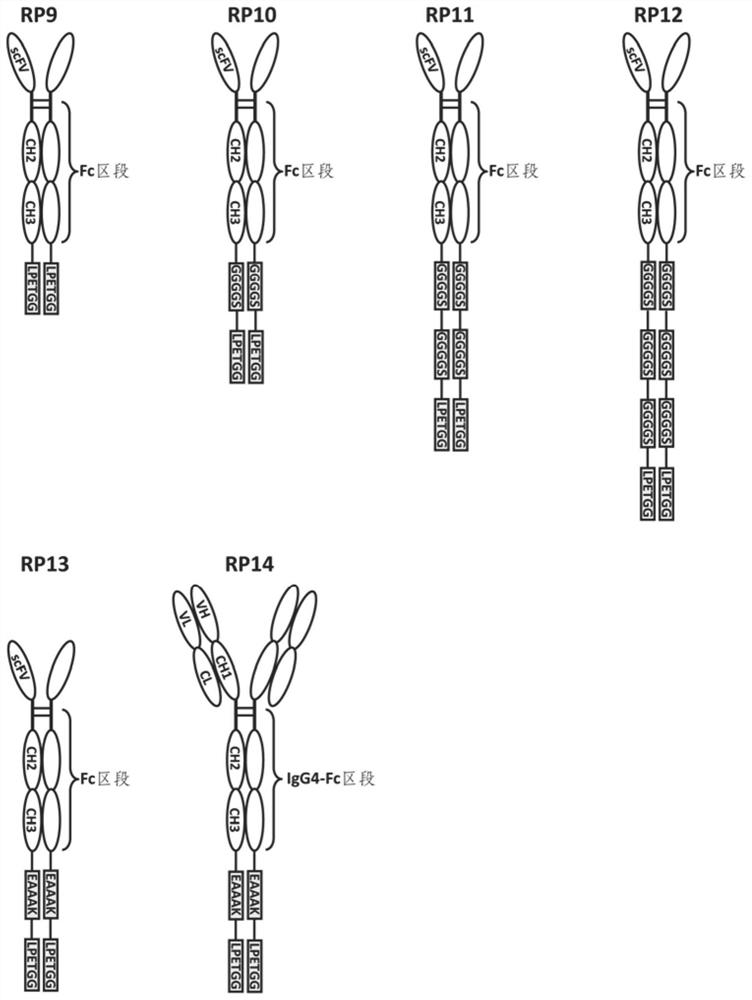
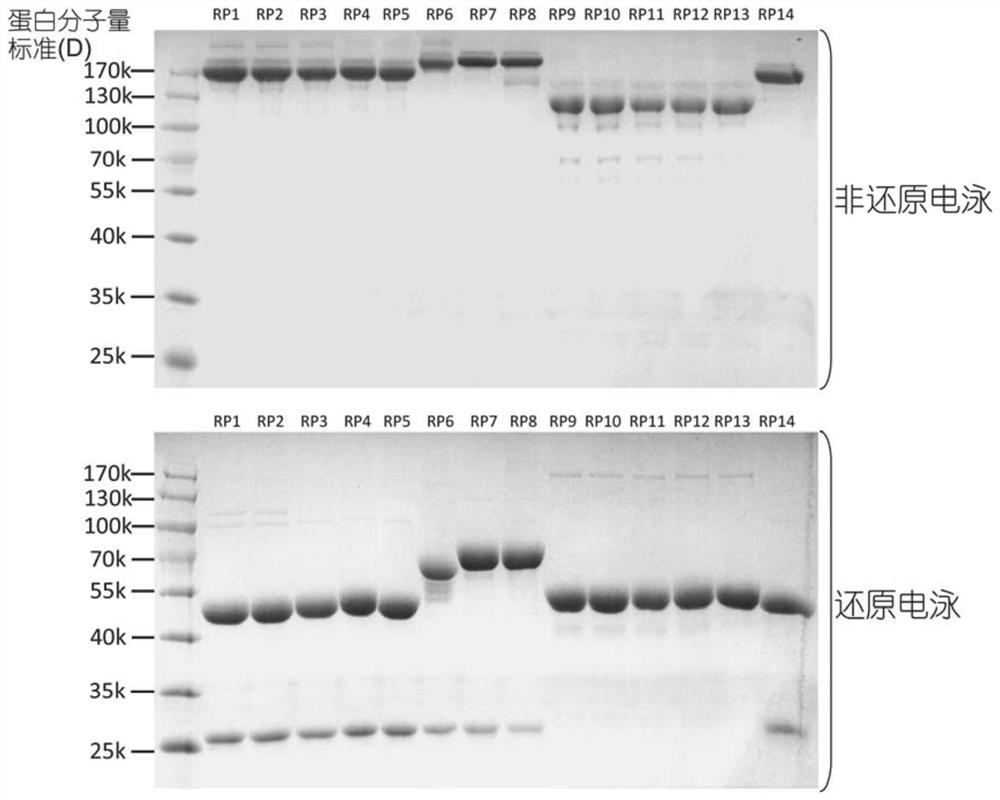
[0099] For the structure of fusion proteins containing Fc segments (including recombinant antibodies and Fc fusion proteins, number: RP1-RP14), see figure 1 . The construction of the fusion protein expression plasmid is as described in Example 1. That is, using the cloning technique described in Example 1, the synthesized nucleic acid was cloned with primers containing HindIII and XhoI restriction sites, and the synthesized sequence was ligated to the vector pCDNA3.1(+) digested with the corresponding enzymes.
[0100] 1.1. Construction of the expression plasmid of recombinant antibody Ab-CH-LPETGG (numbering: RP1):
[0101] The DNA molecule shown in Sequence 3 is used to replace the fragment between the HindIII and XhoI rest...
Embodiment 3
[0224] Example 3 Using transpeptidase A to link a fusion protein containing an Fc segment to NK92-FcγRIII cells and detection of cell activity
[0225] 1. Use transpeptidase A to link the fusion protein containing the Fc segment to NK92-FcγRIII cells (purchased from ATCC, Item No.: pta-8837)
[0226] 1. Take 100 μL of the concentration of 1.0x10 6 / mL of NK92-FcγRIII cell suspension, add transpeptidase A to a final concentration of 20ug / ml, and add a fusion protein containing an Fc segment to a final concentration of 10ug / ml to obtain an incubation system. The incubation system was incubated at 25° C. for 90 min. Centrifuge, collect the cells, and wash thoroughly with pH 7.4, 0.01mol / L PBS buffer. The cells obtained above were named: recombinant protein-SrtA-NK.
[0227] 2. Take 100 μL of the concentration of 1.0x10 6 / mL of NK92-FcγRIII cell suspension, only the fusion protein containing the Fc segment was added to a final concentration of 10ug / ml to obtain an incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com