A betulinic acid prodrug micelle with dual response to reduction and near-infrared light, preparation method and application
A dual-response, near-infrared light technology, applied in the field of medicine, can solve the problems of fast systemic clearance, poor water solubility, non-selective cytotoxicity, etc., and achieve the effect of reducing the use of excipients, improving safety, and increasing drug loading.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
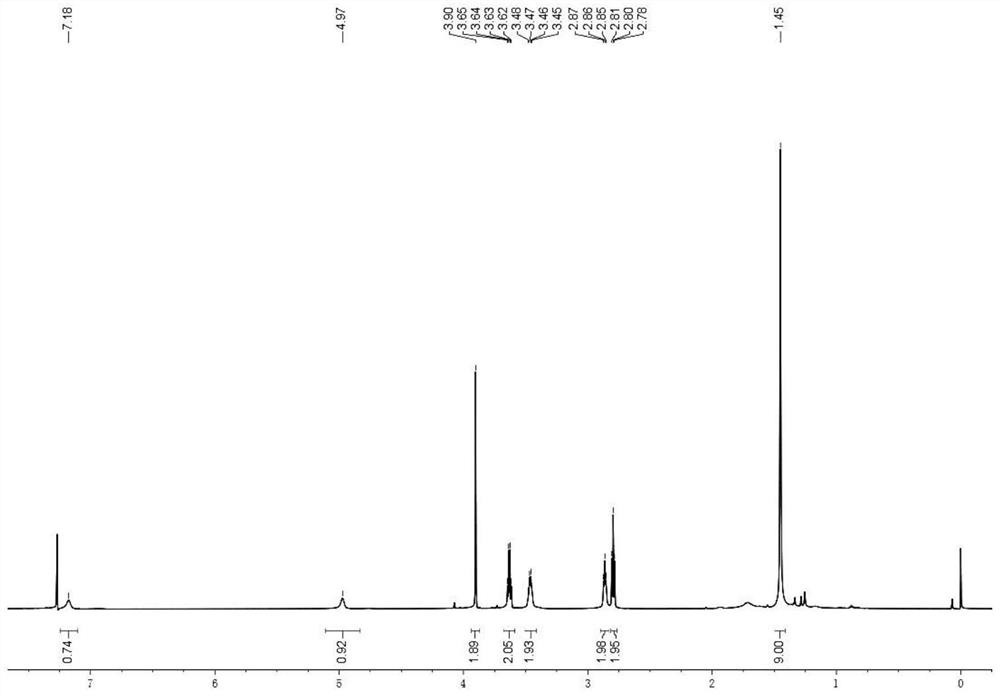
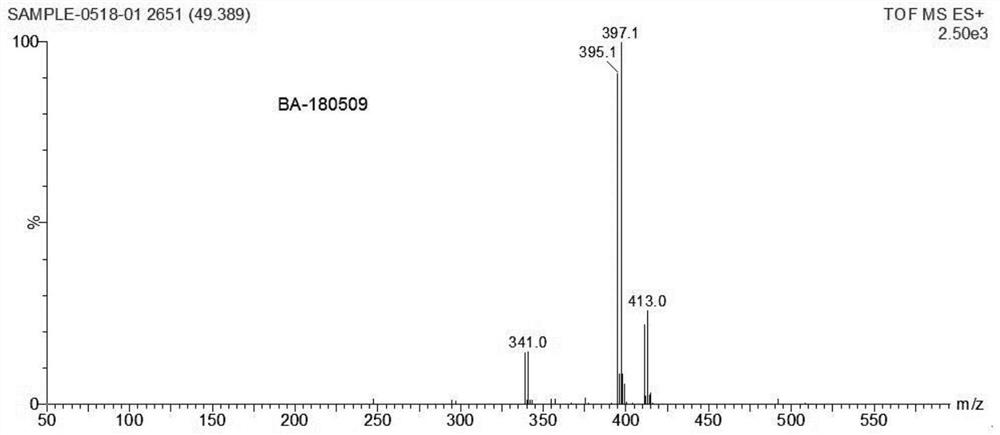
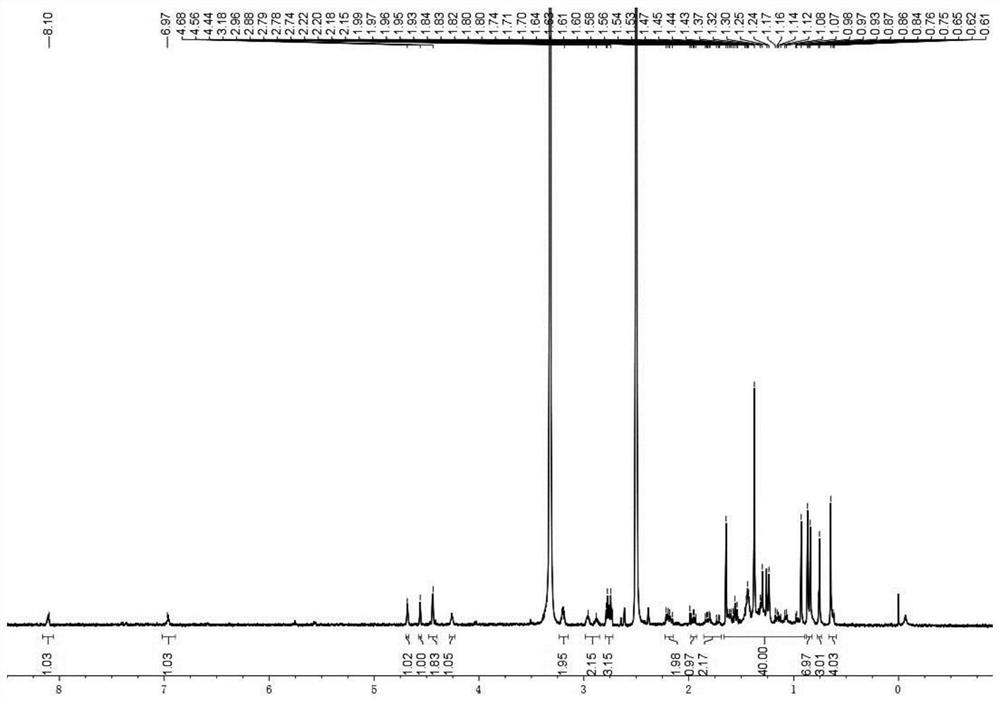
[0064] Preparation of polymer 1 (mPEG 2K -SS-BA Ploymers), the reaction process is referring to the above formula 5:
[0065] H1: To synthesize the compound Link SS, dissolve bromoacetic acid (2.00g, 14.39mmol) in 10mL tetrahydrofuran (THF), add N-hydroxysuccinimide (NHS) (1.84g, 15.83mmol), stir to dissolve Under nitrogen protection, lower the temperature to 0°C; weigh dicyclohexylcarbodiimide (DCC) (3.07g, 15.83mmol) and dissolve it in 10mL THF, add it dropwise to the above reaction solution, and control the rate of addition to ensure that the temperature does not exceed 5 ℃, after dropping, stir at room temperature for 6h; Stir overnight, TLC monitors the complete conversion of raw materials (developing solvent, n-hexane:ethyl acetate=3:1), the reaction solution is filtered, a small amount of THF rinses the filter cake, concentrates the filtrate, and column chromatography (developing solvent, n-hexane:ethyl acetate=3:1) Ester=4:1), to obtain off-white solid compound Link ...
Embodiment 2
[0070] Preparation of polymer 1 (mPEG 5K -SS-BAPloymers), the method of operation is the same as in Example 1, the difference is that in step H3, methoxypolyethylene glycol succinimide ester (mPEG) with a molecular weight of 5000 is used 5K -NHS) to replace 2000 methoxypolyethylene glycol succinimide ester (mPEG 2K -NHS), to obtain white solid polymer 1 (mPEG 5K -SS-BA Ploymers) (2.24g).
Embodiment 3
[0072] Preparation of polymer 1 (mPEG 10K -SS-BAPloymers), the method of operation is the same as in Example 1, the difference is that in step H3, methoxypolyethylene glycol succinimide ester (mPEG) with a molecular weight of 10000 is used 10K -NHS) to replace methoxypolyethylene glycol succinimide ester (mPEG) with a molecular weight of 2000 2K -NHS), to obtain white solid polymer 1 (mPEG 10K -SS-BAPloymers) (1.24g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com