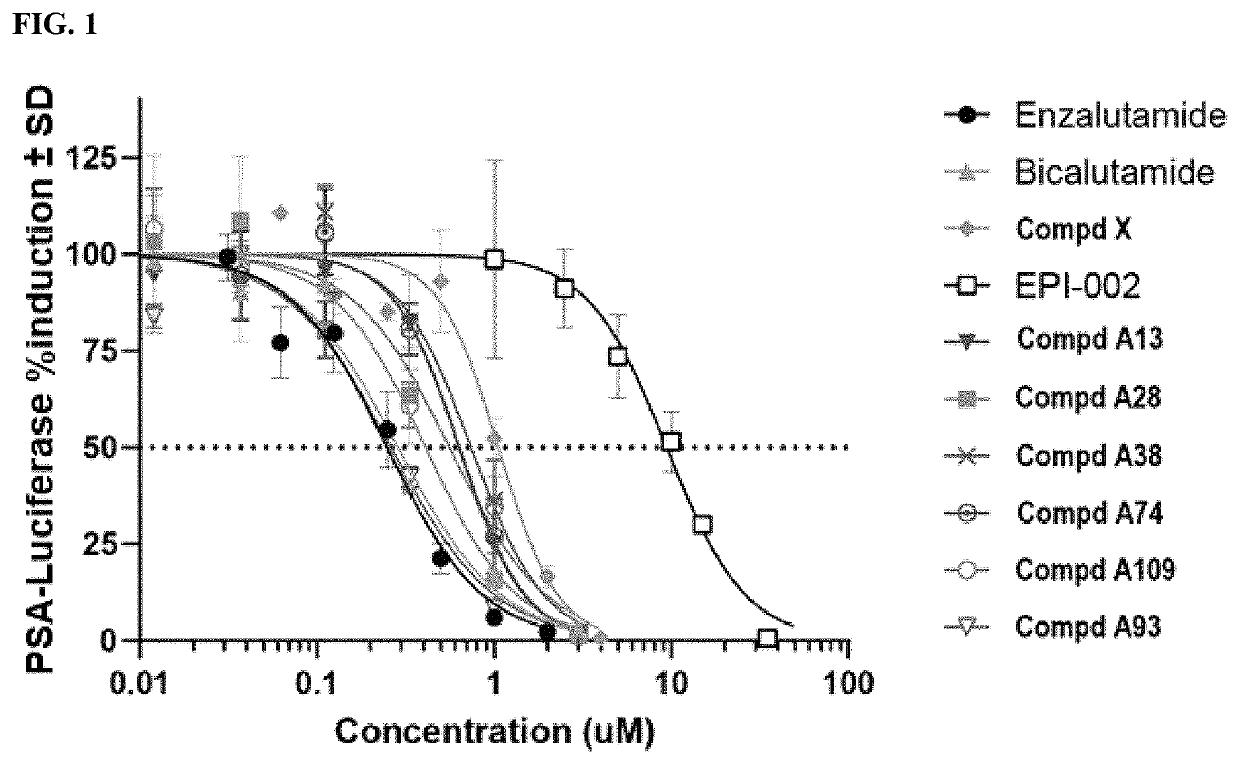
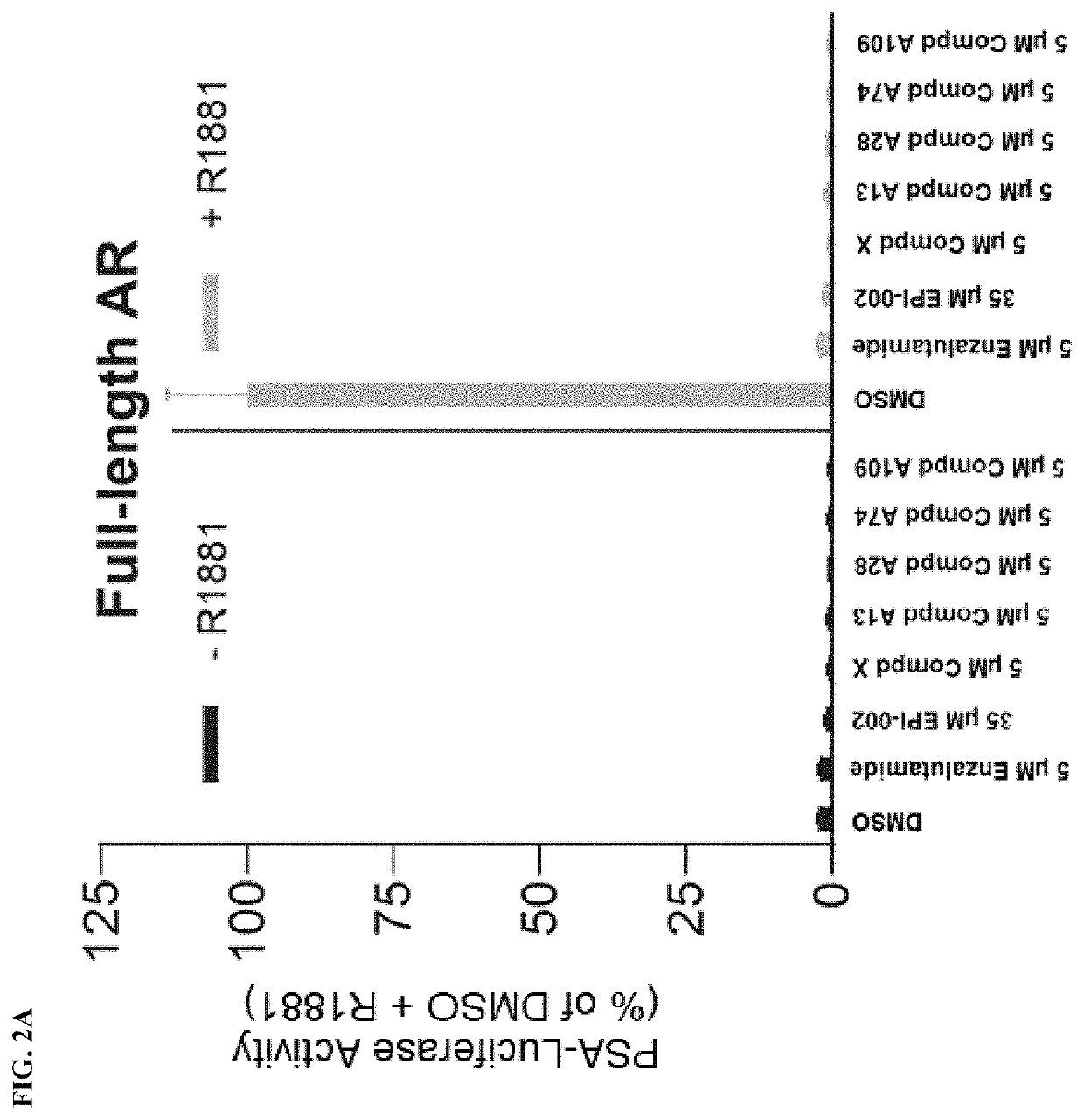
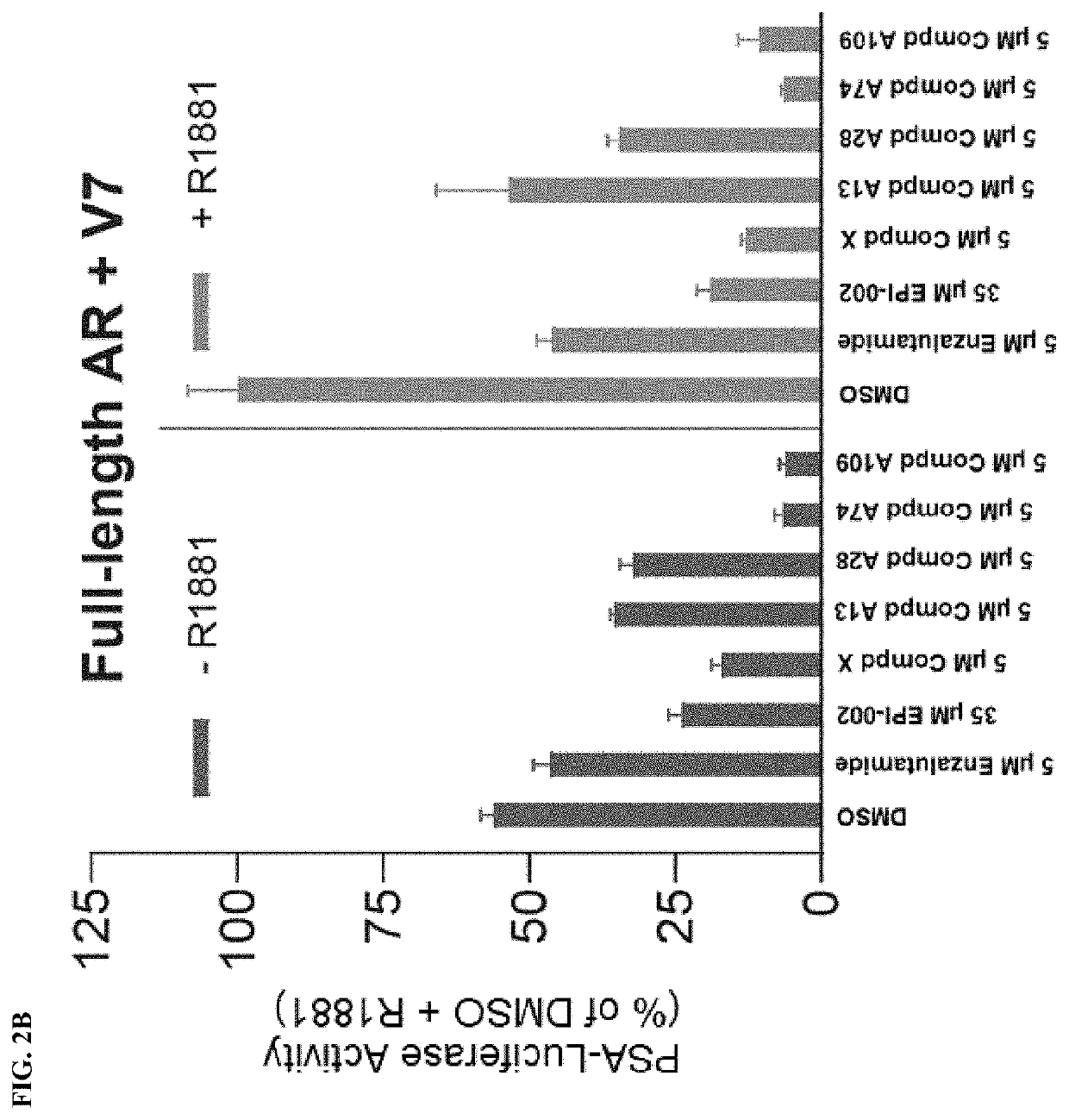
Androgen receptor modulators and methods for their use
a technology of androgen receptors and modulators, applied in the field of tricyclic compounds, can solve problems such as virtual docking drug discovery approaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of 5-[[4-[1-[3,5-dichloro-4-(3-chloropropoxy)phenyl]-1-methyl-ethyl]phenoxy]methyl]-4-methylsulfonyl-oxazole (A3)
[0962]
(4-Methylsulfonyloxazol-5-yl) 4-methylbenzenesulfonate (2)
[0963]To a solution of 4-methylsulfonyloxazol-5-ol (1) (0.10 g, 0.56 mmol), TEA (0.11 g, 1.1 mmol) and DMAP (14 mg, 0.11 mol) in DCM (3 mL) was added Tosyl chloride (0.10 g, 0.52 mmol) dropwise at 0° C. Then the solution was stirred at the same temperature for 1 hour. TLC showed the reaction was completed. The mixture was poured into H2O (5 mL), extracted with DCM (3 mL×3). The combined organic layers were washed with brine (5 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure to give (4-methylsulfonyloxazol-5-yl) 4-methylbenzenesulfonate (2) (0.11 g, yield: 56.6%) as yellow oil. 1H NMR (400 MHz, CHCl3-d) δ 7.98 (s, 1H), 7.93 (d, J=8.40 Hz, 2H), 7.42 (d, J=8.80 Hz, 2H), 4.95 (s, 2H), 3.22 (s, 3H).
Methyl 3,5-dichloro-4-(3-chloropropoxy)benzoate (5)
[0964]To a mixture of methyl 3, 5-dichlo...
example 2
of 4-((4-(2-(3,5-dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenoxy) methyl)-1-(methylsulfonyl)-1H-imidazole (A5)
[0968]
(1-Trityl-1H-imidazol-4-yl)methanol (2)
[0969]To a solution of (1H-imidazol-4-yl)methanol (0.5 g, 5.3 mmol) and Et3N (1.1 g, 10.2 mmol) in DCM (20 mL) was added TrCl (1.5 g, 5.3 mmol) and the mixture was stirred at 50° C. for 4 hours. TLC showed the reaction was completed. The reaction was cooled down, quenched with water (40 mL), extracted with DCM (5 mL×3). The combined organic layers were washed with brine (10 mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by silica-gel column chromatography to give (1-trityl-1H-imidazol-4-yl)methanol (2) (1.0 g, yield: 76.8%) as colorless oil. 1H NMR (400 MHz, CHCl3-d) δ=7.37-7.44 (m, 9H), 7.29 (d, J=1.34 Hz, 2H), 7.10 (dd, J=8.01, 1.41 Hz, 6H), 4.87 (t, J=5.56 Hz, 1H), 4.33 (d, J=5.50 Hz, 2H).
4-(Chloromethyl)-1-trityl-1H-imidazole (3)
[0970]To a solution of (1-tritylimidazol...
example 3
of 2-((4-(2-(3,5-dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenoxy)methyl)-5-(methylsulfonyl)-1, 3, 4-oxadiazole (A7)
[0974]
Methyl 2-(4-(2-(3,5-dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenoxy)acetate (3)
[0975]To a solution of 4-(2-(3,5-dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenol (1) (1.00 g, 2.6 mmol) and methyl 2-bromoacetate (2) (0.49 g, 3.2 mmol) in DMF (10 mL) was added Cs2CO3 (1.30 g, 4.0 mmol) at 25° C. The mixture was stirred at 50° C. for 4 hours. LCMS showed the reaction was completed. The mixture was quenched with water (20 mL) and extracted with EtOAc (20 mL×3). The combined organic layers were washed with brine (20 mL×5), dried over Na2SO4, filtered and concentrated under reduced pressure to give the methyl 2-(4-(2-(3,5-dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenoxy)acetate (3) (1.00 g, yield: 70%) as colorless oil. LCMS (M+23) m z: calcd 444.07. found 467.1.
2-(4-(2-(3,5-Dichloro-4-(3-chloropropoxy)phenyl)propan-2-yl)phenoxy) acetohydrazide (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com